The efficacy of CABLIVI for the treatment of adult patients with acquired thrombotic thrombocytopenic purpura (aTTP) in combination with plasma exchange and immunosuppressive therapy was established in a pivotal multicenter, randomized, double-blind, placebo-controlled trial (HERCULES) (NCT02553317).
A total of 145 patients were enrolled in the HERCULES study; the median age was 45 (range: 18 to 79) years, 69% were female, 73% were White. Patients were randomized to either CABLIVI (n=72) or placebo (n=73). Patients in both groups received plasma exchange and immunosuppressive therapy. Patients were stratified according the severity of neurological involvement (Glasgow Coma Scale score ≤12 or 13 to 15). Patients with sepsis, infection with
Patients received a single 11 mg CABLIVI bolus intravenous injection or placebo prior to the first plasma exchange on study, followed by a daily subcutaneous injection of 11 mg CABLIVI or placebo after completion of plasma exchange, for the duration of the daily plasma exchange period and for 30 days thereafter. If after the initial treatment course, sign(s) of persistent underlying disease such as suppressed ADAMTS13 activity levels remained present, treatment was extended for 7 day intervals for a maximum of 28 days.
The median treatment duration with CABLIVI was 35 days.
The clinical trial protocol specified the CABLIVI dose as 10 mg, to be delivered by withdrawing all of the reconstituted solution from the vial and administering the full amount. A dose recovery study showed that the mean dose that can be withdrawn from a vial is 11 mg. Therefore, based on the dose recovery study, the mean dose delivered in the trial was 11 mg.
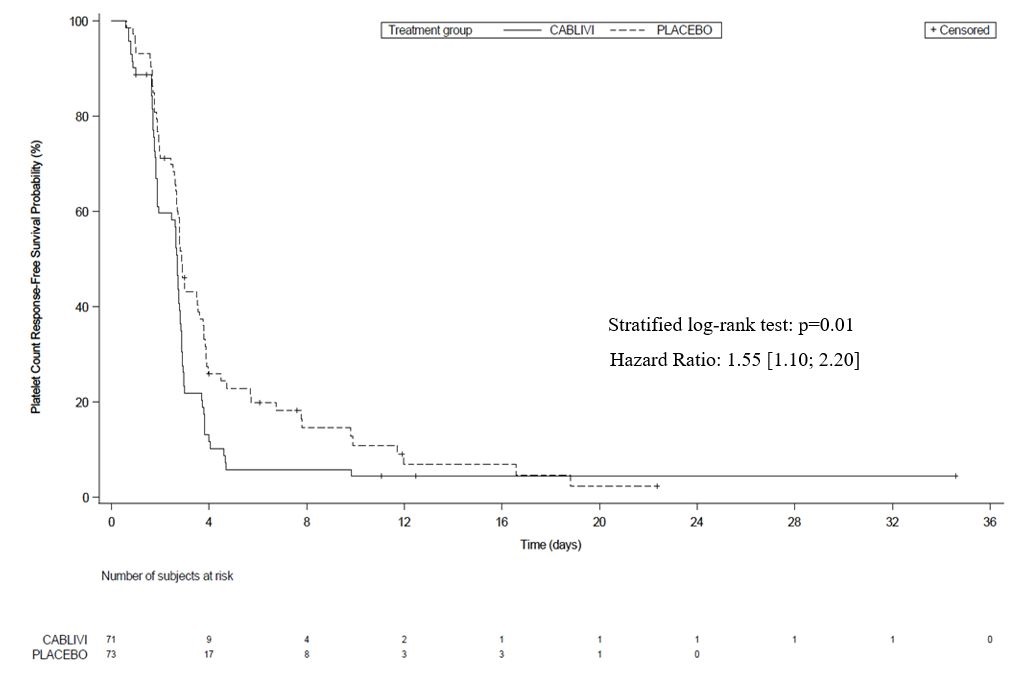
The efficacy of CABLIVI in patients with aTTP was established based on time to platelet count response (platelet count ≥150,000/µL followed by cessation of daily plasma exchange within 5 days). Time to platelet count response was shorter among patients treated with CABLIVI, compared to placebo.
Figure 1: Platelet Response over Time
Treatment with CABLIVI resulted in a lower number of patients with TTP-related death, recurrence of TTP, or at least one treatment-emergent major thromboembolic event (a composite endpoint) during the treatment period (see
The proportion of patients with a recurrence of TTP in the overall study period (the drug treatment period plus the 28-day follow-up period after discontinuation of drug treatment) was lower in the CABLIVI group (9/72 patients [13%]) compared to the placebo group (28/73 patients [38%] (p<0.001). In the 6 patients in the CABLIVI group who experienced a recurrence of TTP during the follow-up period (i.e., a relapse defined as recurrent thrombocytopenia after initial recovery of platelet count (platelet count ≥150,000/µL) that required reinitiation of daily plasma exchange, occurring after the 30-day post daily plasma exchange period), ADAMTS13 activity levels were <10% at the end of the study drug treatment, indicating that the underlying immunological disease was still active at the time CABLIVI was stopped.