Xeljanz
What is Xeljanz (Tofacitinib)?
Xeljanz (tofacitinib) is a prescription medication used to treat several autoimmune and inflammatory conditions, including rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and certain forms of juvenile arthritis. It belongs to a newer class of medications called Janus kinase (JAK) inhibitors, which help reduce inflammation by targeting the immune response at its source. For many patients who do not respond well to traditional therapies, Xeljanz offers a convenient oral option that can improve symptoms and quality of life.
What is Xeljanz?
Xeljanz is the brand name for tofacitinib, developed by Pfizer. It was first approved by the U.S. Food and Drug Administration (FDA) in 2012 to treat adults with moderate to severe rheumatoid arthritis who did not respond well to methotrexate or other disease-modifying antirheumatic drugs (DMARDs) (FDA, 2018). Since then, its approval has expanded to include:
- Psoriatic arthritis (in adults who have not responded adequately to other medications)
- Ulcerative colitis (for moderate to severe cases)
- Polyarticular-course juvenile idiopathic arthritis (pcJIA) in children aged 2 years and older
It’s available as Xeljanz (taken twice daily) and Xeljanz XR (an extended-release tablet taken once daily).
What does Xeljanz do?
Xeljanz helps control chronic inflammation caused by an overactive immune system. In autoimmune diseases, the immune system mistakenly attacks healthy tissues, leading to pain, swelling, stiffness, and fatigue.
Doctors may prescribe Xeljanz when:
- Standard treatments such as methotrexate or biologics are not effective or cause side effects.
- Patients prefer oral medications instead of injectable biologics.
- There’s a need to prevent further joint or intestinal damage due to persistent inflammation.
How effective is Xeljanz? Clinical studies show that Xeljanz can significantly reduce joint pain and swelling, and many patients begin noticing improvement in symptoms within a few weeks of starting treatment (NIH, 2023). In ulcerative colitis, it can help control flare-ups and maintain remission for many individuals.
By managing inflammation effectively, Xeljanz can help preserve long-term joint function and prevent further damage, a key goal in chronic autoimmune conditions.
How does Xeljanz work?
Xeljanz belongs to a class of drugs called JAK inhibitors. It works by targeting enzymes known as Janus kinases (JAK1, JAK2, JAK3, and TYK2), key messengers that transmit immune system signals inside cells.
In autoimmune conditions, these JAK pathways are overactive, leading to excessive inflammation and tissue damage. By partially blocking them, Xeljanz slows down the overactive immune response, helping reduce pain, swelling, and stiffness.
Because it directly affects immune activity, doctors closely monitor patients with regular lab tests. These often include liver enzymes, cholesterol levels, and blood counts to ensure the drug remains safe and effective over time (Mayo Clinic, 2023).
Xeljanz side effects
Like all medications, Xeljanz can cause side effects, though many are mild and manageable. Some side effects may appear early in treatment, while others develop with long-term use.
Common side effects include:
- Headache
- Diarrhea or stomach upset
- Nasal congestion or sore throat
- Mild upper respiratory infections
- Increased cholesterol levels
Less common or serious side effects:
- Shingles (herpes zoster) infection
- Blood clots in the lungs or legs (especially at higher doses)
- Serious infections due to reduced immune activity
- Liver test abnormalities
- Low white or red blood cell counts
Who should not take Xeljanz? Xeljanz may not be suitable for people with active infections, severe liver disease, or a history of blood clots or certain cancers. It is not typically recommended during pregnancy or breastfeeding unless specifically advised by a doctor.
When to seek medical help:
- Shortness of breath, chest pain, or swelling in the legs
- Fever, chills, or persistent cough
- Yellowing of the skin or eyes, unusual bruising, or severe fatigue
The FDA includes a boxed warning about an increased risk of serious infections, heart-related events, cancer, and blood clots associated with JAK inhibitors like Xeljanz (FDA, 2021). Close medical supervision helps minimize these risks.
Xeljanz dosage
Xeljanz is taken by mouth, usually with or without food. The specific dosage depends on the condition being treated, patient age, and individual response to therapy.
- For rheumatoid and psoriatic arthritis, it may be prescribed alone or with methotrexate.
- For ulcerative colitis, it’s often used after other drugs (like TNF inhibitors) fail to provide relief.
Monitoring: Regular medical checkups are essential during Xeljanz treatment. Doctors typically monitor:
- Liver function tests
- Blood counts
- Cholesterol levels
- Signs of infection or blood clots
These tests help ensure the medication continues to work safely. Patients should also update their doctor about any new medications, as some drugs can interact with Xeljanz.
Does Xeljanz have a generic version?
As of 2025, there is no FDA-approved generic version of Xeljanz available in the United States. However, international versions of tofacitinib may exist in other markets.
Both Xeljanz and Xeljanz XR contain the same active ingredient and offer equivalent clinical benefits. The choice between them often depends on patient preference for once-daily versus twice-daily dosing.
Patients should only obtain Xeljanz from licensed pharmacies, as unverified “generic” products online may be counterfeit or unsafe.
Conclusion
Xeljanz (tofacitinib) is a modern treatment option for several autoimmune diseases, helping control inflammation and prevent long-term joint or intestinal damage. Many patients experience noticeable symptom relief within weeks, and ongoing monitoring ensures continued safety and effectiveness.
Because individual responses vary, regular follow-up appointments allow doctors to adjust to treatment as needed. This personalized care helps achieve the best outcomes while minimizing risks.
When taken exactly as prescribed and supported by close medical supervision, Xeljanz can be a valuable therapy that improves daily function, reduces pain, and helps patients live more comfortably with chronic autoimmune conditions.
References
- U.S. Food and Drug Administration (FDA). (2021). https://www.fda.gov
- Mayo Clinic. (2023). Tofacitinib (Oral Route) Description and Brand Names. https://www.mayoclinic.org
- National Institutes of Health (NIH). (2023). Tofacitinib: MedlinePlus Drug Information. https://medlineplus.gov
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- Serious Infections
- Increased Risk of Mortality
- Malignancy and Lymphoproliferative Disorders
- Major Adverse Cardiovascular Events
- Thrombosis
- Gastrointestinal Perforations
- Hypersensitivity Reactions
- Laboratory Abnormalities
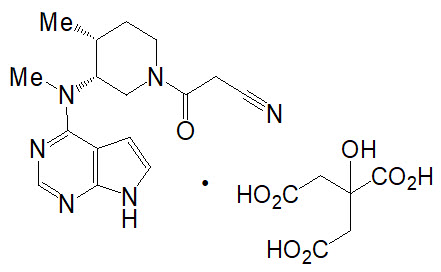
- 5 mg white round, immediate-release film-coated tablet. Each tablet contains 5 mg of tofacitinib (equivalent to 8.08 mg of tofacitinib citrate) and the following inactive ingredients: croscarmellose sodium, HPMC 2910/Hypromellose 6cP, lactose monohydrate, macrogol/PEG3350, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin.
- 10 mg blue round, immediate-release film-coated tablet. Each tablet contains 10 mg of tofacitinib (equivalent to 16.16 mg of tofacitinib citrate) and the following inactive ingredients: croscarmellose sodium, FD&C Blue #1/Brilliant Blue FCF Aluminum Lake, FD&C Blue #2/Indigo Carmine Aluminum Lake, HPMC 2910/Hypromellose 6cP, lactose monohydrate, macrogol/PEG3350, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin.
- 11 mg pink, oval, extended-release film-coated tablet with a drilled hole at one end of the tablet band. Each tablet contains 11 mg of tofacitinib (equivalent to 17.77 mg tofacitinib citrate) and the following inactive ingredients: cellulose acetate, copovidone, hydroxyethyl cellulose, hydroxypropyl cellulose, HPMC 2910/Hypromellose, magnesium stearate, red iron oxide, sorbitol, titanium dioxide and triacetin. Printing ink contains, ammonium hydroxide, ferrosoferric oxide/black iron oxide, propylene glycol, and shellac glaze.
- 22 mg beige, oval, extended-release film-coated tablet with a drilled hole at one end of the tablet band. Each tablet contains 22 mg of tofacitinib (equivalent to 35.54 mg tofacitinib citrate) and the following inactive ingredients: cellulose acetate, copovidone, FD&C Blue #2 Aluminum Lake, hydroxyethyl cellulose, hydroxypropyl cellulose, HPMC 2910/Hypromellose, magnesium stearate, red iron oxide, sorbitol, titanium dioxide, triacetin, and yellow iron oxide. Printing ink contains ammonium hydroxide, ferrosoferric oxide/black iron oxide, propylene glycol, and shellac glaze.
(tofacitinib)
oral solution
- Store XELJANZ oral solution at room temperature between 68°F to 77°F (20°C to 25°C).
- Always store XELJANZ oral solution in the original bottle and carton to protect from light.
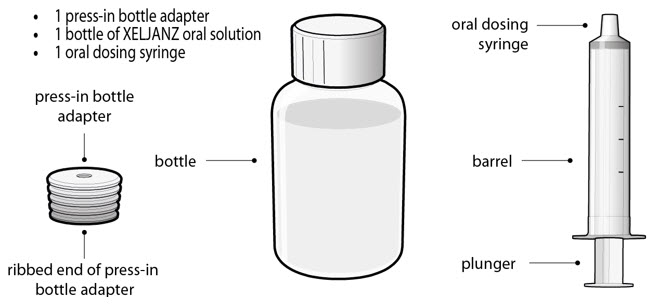
- 1 press-in bottle adapter
- 1 bottle of XELJANZ oral solution
- 1 oral dosing syringe
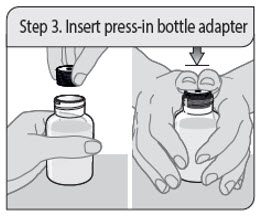
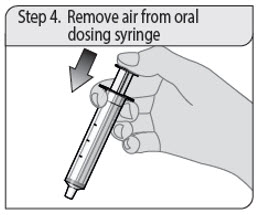
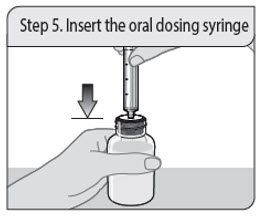
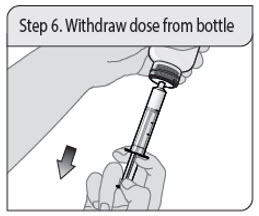
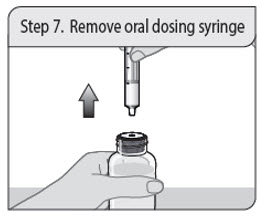
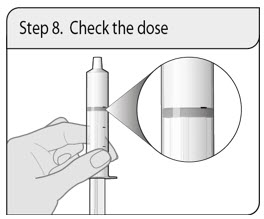
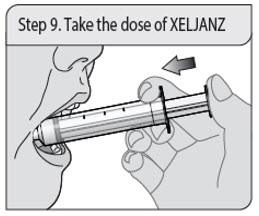
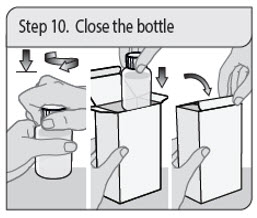
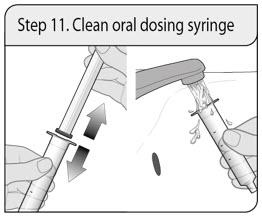

ALWAYS DISPENSE WITH MEDICATION GUIDE
(tofacitinib tablets)
ALWAYS DISPENSE WITH MEDICATION GUIDE
(tofacitinib) tablets

ALWAYS DISPENSE WITH MEDICATION GUIDE
(tofacitinib) tablets
Ulcerative Colitis



- Oral solution bottle
- 1 Oral dosing syringe
- 1 Press-in bottle adapter
- Prescribing Information
- Medication Guide
- Instructions for Use
