Yupelri
What is Yupelri (Revefenacin)?
Chronic Obstructive Pulmonary Disease (COPD) can make something as simple as breathing feel like a constant effort. Patients often describe it as living with a tight chest or struggling to catch their breath even at rest. To help ease these symptoms and improve daily comfort, long-acting bronchodilators play a central role in COPD management. Yupelri (revefenacin) is one such medication designed to provide sustained relief by helping the airways stay open for easier breathing.
Yupelri is a long-acting muscarinic antagonist (LAMA), a type of bronchodilator. It is administered through a nebulizer once daily, making it especially suitable for patients who prefer or require nebulized treatments instead of handheld inhalers. Approved by the U.S. Food and Drug Administration (FDA) in 2018, Yupelri offers a convenient, once-daily option to support long-term COPD symptom control.
What does Yupelri do?
Yupelri is prescribed to help adults with chronic obstructive pulmonary disease (COPD), which includes chronic bronchitis and emphysema, breathe more easily. COPD causes inflammation and narrowing of the airways, leading to persistent coughing, wheezing, and shortness of breath.
By relaxing the airway muscles, Yupelri helps keep the airways open, reducing symptoms like breathlessness and improving lung function over time. Patients using Yupelri as part of a daily maintenance plan often notice better stamina and reduced need for rescue inhalers.
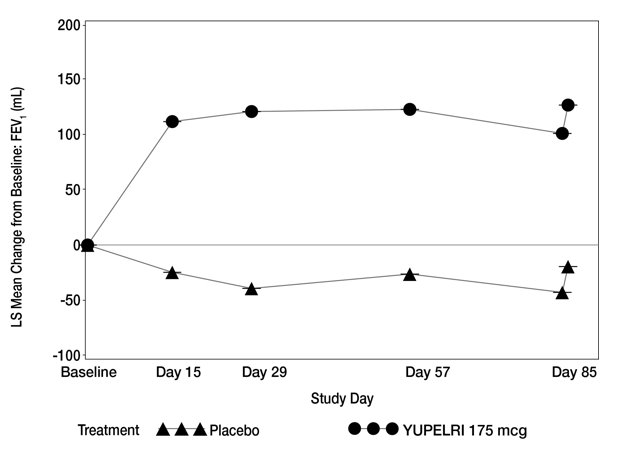
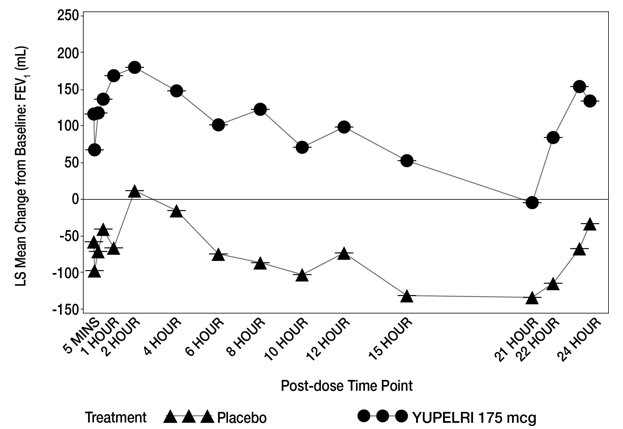
In clinical trials, Yupelri demonstrated significant improvements in lung function, as measured by forced expiratory volume (FEV₁), compared to placebo (FDA, 2024). Many patients report being able to walk farther or perform everyday tasks with less effort when taking this medication consistently.
How does Yupelri work?
Yupelri works by blocking muscarinic (M3) receptors in the smooth muscle of the airways. These receptors are part of the parasympathetic nervous system, which can cause the muscles around the airways to tighten, a process known as bronchoconstriction.
By preventing this tightening, Yupelri allows the muscles to relax, which helps air move more freely in and out of the lungs. This mechanism provides long-lasting bronchodilation that typically lasts for 24 hours after a single dose.
Clinically, this means patients can maintain more stable lung function throughout the day and night, reducing the frequency of flare-ups or hospitalizations related to COPD exacerbations (NIH, 2024).
Yupelri side effects
Like most prescription medications, Yupelri can cause side effects, though not everyone experiences them. Most are mild and manageable, while serious reactions are rare.
Common side effects include:
- Cough or throat irritation after inhalation
- Headache
- Runny or stuffy nose
- Dry mouth
Less common or serious side effects:
- Urinary retention (trouble urinating)
- Fast or irregular heartbeat
- Eye pain or blurred vision
- Allergic reactions such as rash or swelling of the face or tongue
Patients with glaucoma, enlarged prostate, or bladder obstruction should use Yupelri with caution, as it may worsen these conditions.
Seek immediate medical attention if you experience sudden difficulty breathing, severe dizziness, or signs of an allergic reaction. However, most patients tolerate Yupelri well when used as prescribed under medical supervision (Mayo Clinic, 2024).
Yupelri dosage
Yupelri is supplied as a nebulized solution and is taken once daily using a standard jet nebulizer connected to an air compressor. Each dose should be inhaled slowly and deeply through the mouthpiece to ensure proper delivery to the lungs.
It is important not to mix Yupelri with other nebulized medications unless advised by your doctor. Patients should continue other COPD maintenance therapies such as inhaled corticosteroids or long-acting beta-agonists, if prescribed, as Yupelri is not a substitute for those drugs.
Because Yupelri acts locally in the lungs, routine blood tests are not usually required. However, your doctor may monitor lung function, heart rate, and overall symptom control during follow-up visits to ensure the medication is working effectively.
Older adults and those with kidney or liver impairment may require additional monitoring to avoid drug accumulation or side effects.
Does Yupelri have a generic version?
As of 2025, no generic version of Yupelri (revefenacin) is available in the United States. It is currently manufactured by Theravance Biopharma and Viatris.
Since it is still under patent protection, only the branded formulation is sold. However, Yupelri’s manufacturers often offer patient assistance programs or savings cards to help reduce out-of-pocket costs for eligible individuals.
Once a generic version becomes available, it will be held to the same FDA standards of safety, quality, and effectiveness as the brand-name product. Patients can discuss cost-saving options with their healthcare provider or pharmacist.
Conclusion
Yupelri represents a modern advancement in COPD management, offering a convenient, once-daily nebulized option for long-acting symptom control. By helping the airways stay open, it allows patients to breathe more freely, engage in daily activities, and experience a better quality of life.
Although it is not a cure for COPD, Yupelri can significantly reduce breathlessness and improve lung function when used consistently as part of a comprehensive treatment plan that includes lifestyle modifications and pulmonary rehabilitation.
As with all COPD therapies, regular follow-up with your healthcare provider is essential to ensure treatment remains safe and effective. Yupelri is a proven and well-tolerated medication when prescribed and monitored by a qualified medical professional, giving patients confidence in managing their condition and maintaining independence for the long term.
References
- U.S. Food and Drug Administration (FDA). (2024). Yupelri (revefenacin) Prescribing Information. https://www.fda.gov/
- Mayo Clinic. (2024). Chronic Obstructive Pulmonary Disease (COPD): Treatment Overview. https://www.mayoclinic.org/
- MedlinePlus. (2024). Revefenacin Inhalation: Drug Information. https://medlineplus.gov/
- National Institutes of Health (NIH). (2024). COPD: Current Pharmacologic Management. https://www.nih.gov/
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to compare the effectiveness of inhaled bronchodilators delivered via nebulizers vs. dry powder inhalers (DPIs) in symptomatic participants with Chronic Obstructive Pulmonary Disease (COPD) who have airflow obstruction (FEV1/FVC ≤ 70%) and show significant air trapping (RV ≥ 120% of predicted). The investigators hypothesize that, in patients with symptomatic COPD, ther...
Related Latest Advances
Brand Information
- Paradoxical bronchospasm
- Worsening of narrow-angle glaucoma
- Worsening of urinary retention
- Immediate hypersensitivity reactions [
- Store YUPELRI in the protective foil pouch.
- Store at room temperature from 68°F to 77°F (20°C to 25°C); excursions permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Protect from direct sunlight and excessive heat.
- Discard any solution that is not clear and colorless.
- YUPELRI should only be administered via a standard jet nebulizer connected to an air compressor with an adequate airflow, and equipped with a mouthpiece.
- Do not swallow or inject YUPELRI.
- Decreasing effectiveness of inhaled, short-acting beta
- Need for more inhalations than usual of inhaled, short-acting beta
- Significant decrease in lung function as outlined by the physician
© 2021 Viatris Inc.
YUPELRI® is a registered trademark of Mylan Specialty L.P., a Viatris Company.
Rx only
(revefenacin) inhalation solution
175 mcg/3 mL
unit-dose vials
Each vial contains 175 mcg of revefenacin in an isotonic, sterile aqueous solution containing sodium chloride, citric acid and sodium citrate. Hydrochloric acid or sodium hydroxide may be used to adjust the pH.
Store YUPELRI (revefenacin) inhalation solution in the protective foil pouch. Store at room temperature from 68°F to 77°F (20°C to 25°C); excursions permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Protect from direct sunlight and excessive heat. The YUPELRI unit-dose vial should only be removed from the foil pouch and opened IMMEDIATELY BEFORE USE. The vial and any residual content should be discarded after use. Discard any solution that is not clear and colorless. Use only as directed by your healthcare provider.


