Brand Name
Fetzima
Generic Name
Levomilnacipran
View Brand Information FDA approval date: July 25, 2013
Classification: Serotonin and Norepinephrine Reuptake Inhibitor
Form: Kit, Capsule
What is Fetzima (Levomilnacipran)?
Levomilnacipran is indicated for the treatment of major depressive disorder in adults. Limitation of Use : Levomilnacipran extended-release capsules are not approved for the management of fibromyalgia. The efficacy and safety of levomilnacipran extended-release capsules for the management of fibromyalgia have not been established. Levomilnacipran is a serotonin and norepinephrine reuptake inhibitor indicated for the treatment of Major Depressive Disorder in adults. Limitation of Use : Levomilnacipran extended-release capsules are not approved for the management of fibromyalgia. The efficacy and safety of levomilnacipran extended-release capsules for the management of fibromyalgia have not been established.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Fetzima (LEVOMILNACIPRAN HYDROCHLORIDE)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)].
1INDICATIONS AND USAGE
FETZIMA
Limitation of Use: FETZIMA is not approved for the management of fibromyalgia. The efficacy and safety of FETZIMA for the management of fibromyalgia have not been established.
2DOSAGE FORMS AND STRENGTHS
FETZIMA (levomilnacipran) is available as 20 mg, 40 mg, 80 mg, and 120 mg extended-release capsules.
3CONTRAINDICATIONS
FETZIMA is contraindicated:
- in patients with hypersensitivity to levomilnacipran, milnacipran HCl, or to any excipient in the formulation.
- with the use of MAOIs intended to treat psychiatric disorders with FETZIMA or within 7 days of stopping treatment with FETZIMA is contraindicated because of an increased risk of serotonin syndrome. The use of FETZIMA within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated
Starting FETZIMA in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label.
- Hypersensitivity
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults
- Serotonin Syndrome
- Elevated Blood Pressure
- Elevated Heart Rate
- Increased Risk of Bleeding
- Angle Closure Glaucoma
- Urinary Hesitation or Retention
- Activation of Mania/Hypomania
- Seizure
- Discontinuation Syndrome
- Hyponatremia
- Sexual Dysfunction
4.1Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Patient exposure
The safety of FETZIMA was evaluated in 3,317 patients (18 to 78 years of age) diagnosed with MDD who participated in clinical studies, representing 1,186 patient-years of exposure. Among the 3,317 FETZIMA-treated patients, 1,583 were exposed to FETZIMA in short-term, placebo-controlled studies. There were 825 patients who continued from short-term studies into a one-year, open-label extension study.
Of the 3,317 patients exposed to at least one dose of FETZIMA, 895 patients were exposed to FETZIMA for at least 6 months and 367 were exposed for one year. In these studies, FETZIMA was given at doses ranging from 40 mg to 120 mg once daily and was given without regard to food.
Adverse reactions reported as reasons for discontinuation of treatment
In the short-term placebo-controlled pre-marketing studies for MDD, 9% of the 1,583 patients who received FETZIMA (40 mg to 120 mg) discontinued treatment due to an adverse reaction, compared with 3% of the 1,040 placebo-treated patients in those studies. The most common adverse reaction leading to discontinuation in at least 1% of the FETZIMA-treated patients in the short-term placebo-controlled studies was nausea (1.5%).
Common adverse reactions in placebo-controlled MDD studies
The most commonly observed adverse reactions in FETZIMA-treated MDD patients in placebo-controlled studies (incidence ≥ 5% and at least twice the rate of placebo) were: nausea, constipation, hyperhidrosis, heart rate increased, erectile dysfunction, ejaculation disorder, tachycardia, vomiting, and palpitations.
Table 3 shows the incidence of adverse reactions that occurred in ≥ 2% of FETZIMA-treated MDD patients and at least twice the rate of placebo in the placebo-controlled studies.
Dose-related adverse reactions
In pooled data from the short-term placebo-controlled fixed-dose studies, there were no dose-related adverse reactions (greater than 2% overall incidence) in patients treated with FETZIMA across the dose range 40 mg to 120 mg once daily, with the exception of erectile dysfunction and urinary hesitation (see Table 4).
Other adverse reactions observed in clinical studies
Other infrequent adverse reactions, not described elsewhere in the label, occurring at an incidence of < 2% in MDD patients treated with FETZIMA were:
Cardiac disorders: Angina pectoris; Supraventricular and Ventricular extrasystoles
Eye disorders: Dry eye; Vision blurred; Conjunctival hemorrhage
General disorders: Chest pain; Thirst
Gastrointestinal disorders: Abdominal pain; Flatulence
Investigations disorders: Blood cholesterol increased; Liver function test abnormal
Nervous System disorders: Migraine; Paraesthesia; Syncope; Extrapyramidal disorder
Psychiatric disorders: Agitation; Anger; Bruxism; Panic attack; Tension; Aggression
Renal and Urinary disorder: Pollakiuria; Hematuria; Proteinuria
Respiratory, thoracic and mediastinal disorders: Yawning
Skin and subcutaneous tissue disorders:Dry skin; Pruritus; Urticaria
4.2Postmarketing Experience
The following adverse reaction has been identified during post-approval use of FETZIMA or other selective serotonin and norepinephrine reuptake inhibitors. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders:Takotsubo cardiomyopathy
Respiratory, thoracic and mediastinal disorders: Anosmia, Hyposmia
5DESCRIPTION
FETZIMA contains levomilnacipran, a selective serotonin and norepinephrine reuptake inhibitor (SNRI), in the form of hydrochloride salt with the chemical name of levomilnacipran hydrochloride is (1S,2R)-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide hydrochloride. Its empirical formula is C
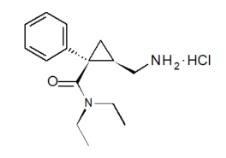
FETZIMA extended-release capsules are intended for oral administration. Each FETZIMA capsule contains 23.0, 45.9, 91.8, or 137.8 mg of levomilnacipran hydrochloride equivalent to 20, 40, 80, or 120 mg of levomilnacipran, respectively.
Inactive ingredients include ethylcellulose, hypromellose, povidone, sugar spheres, talc, titanium dioxide, and triethyl citrate. Inactive ingredients also include black iron oxide, red iron oxide (80 mg and 120 mg capsules only), shellac glaze, and yellow iron oxide (20 mg and 40 mg capsules only).
6HOW SUPPLIED/STORAGE AND HANDLING
FETZIMA extended-release capsules are supplied in the following configurations:
FETZIMA Titration Pack is supplied in the following configuration:
Storage and Handling
All package configurations: Store at 25°C (77°F); excursions permitted between 15°C and 30°C (59°F and 86°F)
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Suicide Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidal thoughts and behaviors, especially early during treatment and when the dose is adjusted up or down, and instruct them to report such symptoms to the healthcare provider
Dosing and Administration
Advise patients that FETZIMA should be swallowed whole and should not be chewed, crushed, or opened.
Advise patients that FETZIMA can be taken with or without food.
Instruct patients if they miss a dose, to take the missed dose as soon as they remember. If it is almost time for the next dose, instruct them to skip the missed dose and take their next dose at the regular time. Advise them not to take two doses of FETZIMA at the same time.
Concomitant Medication
Instruct patients not to take FETZIMA with an MAOI or within 14 days of stopping an MAOI and to allow 7 days after stopping FETZIMA before starting an MAOI
Allergic Reactions
Advise patients to notify their healthcare provider if they develop an allergic reaction such as rash, hives, swelling, or difficulty breathing
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of FETZIMA with other serotonergic agents (including triptans, tricyclic antidepressants, opioids, lithium, amphetamines, tryptophan, buspirone, and St. John’s Wort supplements)
Elevated Blood Pressure and Heart Rate
Advise patients that they should have regular monitoring of blood pressure and heart rate when taking FETZIMA
Increased Risk of Bleeding
Caution patients about the concomitant use of FETZIMA and NSAIDs, aspirin, warfarin, or other drugs that affect coagulation because combined use has been associated with an increased risk of bleeding. Advise patients to inform their healthcare provider if they are taking or planning to take any prescription or over-the-counter medications that increase the risk of bleeding
Angle Closure Glaucoma
Patients should be advised that taking FETZIMA can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle closure glaucoma
Urinary Hesitation or Retention
Caution patients about the risk of urinary hesitation and retention while taking FETZIMA, particularly in patients prone to obstructive urinary disorders. Instruct patients to consult with their healthcare provider if they develop any problems with urine flow
Activation of Mania/Hypomania
Advise patients and their caregivers to look for signs of activation of mania/hypomania
Seizures
Caution patients about using FETZIMA if they have a history of a seizure disorder
Discontinuation Syndrome
Advise patients not to abruptly stop taking FETZIMA without first talking with their healthcare provider. Patients should be aware that discontinuation effects may occur when suddenly stopping FETZIMA and they should monitor for discontinuation symptoms
Hyponatremia
Advise patients that hyponatremia has been reported as a result of treatment with FETZIMA. Advise patients of the signs and symptoms of hyponatremia
Sexual Dysfunction
Advise patients that use of FETZIMA may cause symptoms of sexual dysfunction in both male and female patients. Inform patients that they should discuss any changes in sexual function and potential management strategies with their healthcare provider
Alcohol
Advise patients to avoid consumption of alcohol while taking FETZIMA
Pregnancy
- Advise pregnant females to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with FETZIMA.
- Advise patients that FETZIMA may increase the risk of neonatal complications requiring prolonged hospitalization, respiratory support, tube feeding.
- Advise patient that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to FETZIMA during pregnancy
Lactation
Advise breastfeeding patients using FETZIMA to monitor infants for sedation, agitation, irritability, poor feeding, and poor weight gain and to seek medical care if they notice these signs
Interference with Cognitive and Motor Performance
Caution patients about operating hazardous machinery, including automobiles, until they are reasonably certain that FETZIMA therapy does not adversely affect their ability to engage in such activities.
Distributed by:
AbbVie, Inc.
©2024 AbbVie. All rights reserved.
20085454
8PRINCIPAL DISPLAY PANEL
Rx only NDC 0456-2220-30
Fetzima®
levomilnacipran
extended-release capsules
20 mg per capsule
Dispense the accompanying
Medication Guide to each patient.
30 capsules
Fetzima®
levomilnacipran
extended-release capsules
20 mg per capsule
Dispense the accompanying
Medication Guide to each patient.
30 capsules

9PRINCIPAL DISPLAY PANEL
Rx only NDC 0456-2240-30
Fetzima®
levomilnacipran
extended-release capsules
40 mg per capsule
Fetzima®
levomilnacipran
extended-release capsules
40 mg per capsule
Dispense the accompanying
Medication Guide to each patient.
30 capsules
Medication Guide to each patient.
30 capsules

10PRINCIPAL DISPLAY PANEL
Rx only NDC 0456-2280-30
Fetzima®
levomilnacipran
extended-release capsules
80 mg per capsule
Dispense the accompanying
Medication Guide to each patient.
30 capsules
Fetzima®
levomilnacipran
extended-release capsules
80 mg per capsule
Dispense the accompanying
Medication Guide to each patient.
30 capsules

11PRINCIPAL DISPLAY PANEL
Rx only NDC 0456-2212-30
Fetzima®
levomilnacipran
extended-release capsules
120 mg per capsule
Fetzima®
levomilnacipran
extended-release capsules
120 mg per capsule
Dispense the accompanying
Medication Guide to each patient.
30 capsules
Medication Guide to each patient.
30 capsules

12PRINCIPAL DISPLAY PANEL
Rx Only NDC 0456-2202-28
Take one 20 mg capsule once daily
On Day 1 and Day 2.
Take one 40 mg capsule once daily
On Day 3 through Day 28.
Patients: Lift up flap to find Instructions for sliding out the tray.
Dispense the accompanying Medication Guide to each patient.

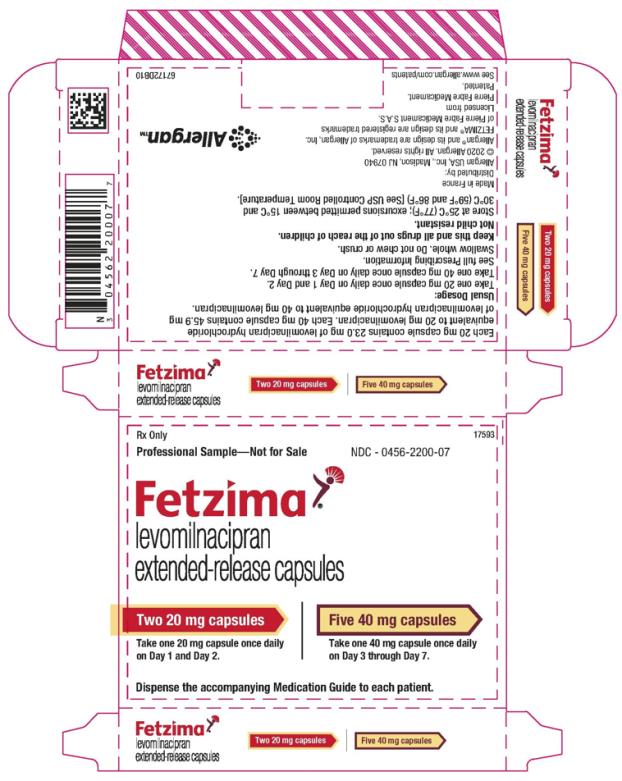
13PRINCIPAL DISPLAY PANEL
Rx Only
NDC 0456-2200-07
Take one 20 mg capsule once daily
On Day 1 and Day 2.
Take one 40 mg capsule once daily
On Day 3 through Day 7.
Dispense the accompanying Medication Guide to each patient.