Ioflupane I-123
What is Ioflupane (Ioflupane I-123)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This study aims to study, in patient with Parkinson's disease, mild to moderate stage (according to Movement Disorder Society Clinical Diagnostic Criteria for Parkinson's Disease, Postuma et al., 2015): * the evolution of oculomotricity markers over time. * the correlation between neurological evaluations (motor and non-motor scores), neuropsychological evaluations (cognitive disorders) and oculom...
Summary: The purpose of this study is to identify and distinguish two different types of Progressive Apraxia of Speech through clinical imaging and testing.
Summary: The study is designed to characterize the clinical, neuropsychological, polysomnographic, and neuroimaging findings among subjects with Alzheimer's disease, Lewy Body dementia, and Parkinsons' Disease.
Related Latest Advances
Brand Information
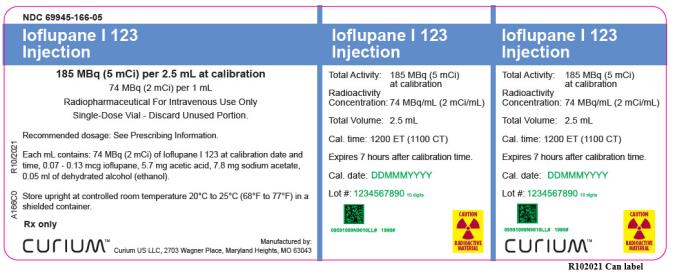
Ioflupane I 123 Injection
185 MBq (5 mCi) per 2.5 mL at calibration
74 MBq (2 mCi) per 1 mL
Ioflupane I 123 Injection
185 MBq (5 mCi) per 2.5 mL at calibration
[74 MBq (2 mCi) per 1 mL]
![PRINCIPAL DISPLAY PANEL
NDC 69945-166-05
Ioflupane I 123 Injection
185 MBq (5 mCi) per 2.5 mL at calibration
[74 MBq (2 mCi) per 1 mL]
Radiopharmaceutical - For Intravenous Use Only
Single-Dose Vial – Discard Unused Portion.
Cal. time:
1200 ET (1100 CT)
Cal. date:
Lot #:
Volume: 2.5 mL
Radioactive Concentration:
74 MBq/mL (2 mCi/mL)
Total Activity:
185 MBq (5 mCi)
Recommended Dosage:
See Prescribing Information
Rx only
Store upright at controlled
room temperature 20°C to
25°C (68°F to 77°F) in a
shielded container. Expires
7 hours after calibration time.
Each mL contains 74 MBq
(2 mCi) of Ioflupane I 123 at
calibration, 0.07 - 0.13 mcg
ioflupane, 5.7 mg acetic acid,
7.8 mg sodium acetate,
0.05 mL ethanol.
CAUTION
RADIOACTIVE MATERIAL
Manufactured by:
Curium US LLC
2703 Wagner Place
Maryland Heights, MO
63043
A166V0 R12/2021](https://dailymed.nlm.nih.gov/dailymed/image.cfm?name=ioflupane-i-123-04.jpg&setid=66d20aee-4530-48dd-a483-ed7604b36d97)


