Generic Name
Oxybate
Brand Names
Lumryz, Xyrem
FDA approval date: July 17, 2002
Classification: Central Nervous System Depressant
Form: Kit, For, Solution
What is Lumryz (Oxybate)?
LUMRYZ is indicated for the treatment of cataplexy or excessive daytime sleepiness in adults with narcolepsy. LUMRYZ is a central nervous system depressant indicated for the treatment of cataplexy or excessive daytime sleepiness in adults with narcolepsy .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
WARNING: CENTRAL NERVOUS SYSTEM (CNS) DEPRESSION AND ABUSE AND MISUSE
Central Nervous System Depression
LUMRYZ (sodium oxybate) is a CNS depressant. Clinically significant respiratory depression and obtundation may occur in patients treated with LUMRYZ at recommended doses
Abuse and Misuse
LUMRYZ (sodium oxybate) is the sodium salt of gamma-hydroxybutyrate (GHB). Abuse or misuse of illicit GHB, either alone or in combination with other CNS depressants, is associated with CNS adverse reactions, including seizure, respiratory depression, decreases in the level of consciousness, coma, and death
Because of the risks of CNS depression and abuse and misuse, LUMRYZ is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the LUMRYZ REMS
1INDICATIONS AND USAGE
LUMRYZ is indicated for the treatment of cataplexy or excessive daytime sleepiness (EDS) in patients 7 years of age and older with narcolepsy.
2DOSAGE FORMS AND STRENGTHS
For extended-release oral suspension: LUMRYZ is a white to off-white powder provided in packets of 4.5 g, 6 g, 7.5 g, or 9 g of sodium oxybate.
3CONTRAINDICATIONS
LUMRYZ is contraindicated for use in:
● combination with sedative hypnotics
● combination with alcohol
● patients with succinic semialdehyde dehydrogenase deficiency
4ADVERSE REACTIONS
The following clinically significant adverse reactions appear in other sections of the labeling:
● CNS Depression
● Abuse and Misuse
● Respiratory Depression and Sleep-Disordered Breathing
● Depression and Suicidality
● Other Behavioral or Psychiatric Adverse Reactions
● Parasomnias
● Use in Patients Sensitive to High Sodium Intake
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adult Patients
LUMRYZ was studied in one placebo-controlled trial (Study 1)
Adverse Reactions Leading to Treatment Discontinuation
In Study 1, 15.9% of patients treated with LUMRYZ discontinued because of adverse reactions, compared to 1.9% of patients receiving placebo. The most common adverse reaction leading to discontinuation was dizziness (4.7%). For LUMRYZ, 5.6% of patients discontinued due to adverse reactions on 4.5 g, 4.1% on 6 g, 4.5% on 7.5 g, and 3.9% on 9 g dose.
Most Common Adverse Reactions
The most common adverse reactions (incidence ≥5% and greater than placebo) reported for any dose of LUMRYZ were nausea, dizziness, enuresis, headache, and vomiting.
Adverse Reactions Occurring at an Incidence of 2% or Greater
Table 2 lists adverse reactions occurring in 2% or more of LUMRYZ-treated patients on any individual dose and at a rate greater than placebo-treated patients in Study 1.
Dose-Response Information
In the clinical trial in adult patients with narcolepsy, a dose-response relationship was observed for enuresis and somnolence.
Additional Adverse Reactions
Adverse reactions observed in clinical studies with immediate-release sodium oxybate (≥2%), but not observed in Study 1 at a frequency of higher than 2%, and which may be relevant for LUMRYZ: diarrhea, abdominal pain upper, dry mouth, pain, feeling drunk, peripheral edema, cataplexy, muscle spasms, pain in extremity, tremor, disturbance in attention, paresthesia, sleep paralysis, disorientation, irritability, and hyperhidrosis.
Pediatric Patients (7 Years of Age and Older)
The safety of LUMRYZ for the treatment of cataplexy or excessive daytime sleepiness in pediatric patients 7 years of age and older with narcolepsy is supported by an adequate and well-controlled trial of immediate-release sodium oxybate
In this trial, 104 patients aged 7 to 17 years (37 patients aged 7 to 11 years; 67 patients aged 12 to 17 years) with narcolepsy received immediate-release sodium oxybate for up to one year. This trial included an open-label safety continuation period in which eligible patients received immediate-release sodium oxybate for up to an additional 2 years. The median and maximum exposure across the entire trial were 371 and 987 days, respectively.
Adverse Reactions Leading to Treatment Discontinuation
In the pediatric clinical trial with immediate-release sodium oxybate, 7 of 104 patients reported adverse reactions that led to withdrawal from the study (hallucination, tactile; suicidal ideation; weight decreased; sleep apnea syndrome; affect lability; anger, anxiety, depression; and headache).
Adverse Reactions in the Pediatric Clinical Trial
The most common adverse reactions (≥5%) were nausea (20%), enuresis (19%), vomiting (18%), headache (17%), weight decreased (13%), decreased appetite (9%), dizziness (8%), and sleepwalking (6%).
Additional information regarding safety in pediatric patients appears in the following sections:
● Respiratory Depression and Sleep-Disordered Breathing
● Depression and Suicidality
● Other Behavioral or Psychiatric Adverse Reactions
● Parasomnias
The overall adverse reaction profile of immediate-release sodium oxybate in the pediatric clinical trial was similar to that seen in the adult clinical trial with immediate-release sodium oxybate. The safety profile in pediatric patients with LUMRYZ is expected to be similar to that of adult patients treated with LUMRYZ and to that of pediatric patients treated with immediate-release sodium oxybate.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of sodium oxybate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Arthralgia, decreased appetite, fall*, fluid retention, hangover, headache, hypersensitivity, hypertension, memory impairment, nocturia, panic attack, vision blurred, and weight decreased.
*The sudden onset of sleep in patients taking sodium oxybate, including in a standing position or while rising from bed, has led to falls complicated by injuries, in some cases requiring hospitalization.
5DESCRIPTION
Sodium oxybate, a CNS depressant, is the active ingredient in LUMRYZ for extended-release oral suspension. The chemical name for sodium oxybate is sodium 4-hydroxybutyrate. The molecular formula is C
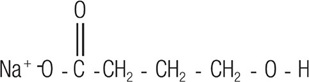
Sodium oxybate is a white to off-white solid powder.
Each packet of LUMRYZ contains 4.5 g, 6 g, 7.5 g, or 9 g of sodium oxybate, equivalent to 3.7 g, 5.0 g, 6.2 g, or 7.4 g of oxybate, respectively. The inactive ingredients are carrageenan, hydrogenated vegetable oil, hydroxyethyl cellulose, magnesium stearate, malic acid, methacrylic acid copolymer, microcrystalline cellulose, povidone, and xanthan gum.
6PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (
Central Nervous System Depression
Inform patients and/or caregivers that LUMRYZ can cause central nervous system depression, including respiratory depression, hypotension, profound sedation, syncope, and death. Instruct patients to not engage in activities requiring mental alertness or motor coordination, including operating hazardous machinery, for at least 6 hours after taking LUMRYZ. Instruct patients and/or caregivers to inform their healthcare providers of all the medications they take
Abuse and Misuse
Inform patients and/or caregivers that the active ingredient of LUMRYZ is gamma-hydroxybutyrate (GHB), which is associated with serious adverse reactions with illicit use and abuse
LUMRYZ REMS
LUMRYZ is available only through a restricted program called the LUMRYZ REMS
● LUMRYZ is dispensed only by pharmacies that are specially certified
● LUMRYZ will be dispensed and shipped only to patients who are enrolled in the LUMRYZ REMS
LUMRYZ is available only from certified pharmacies participating in the program. Therefore, provide patients and/or caregivers with the telephone number and website for information on how to obtain the product.
Alcohol or Sedative Hypnotics
Advise patients and/or caregivers that alcohol and other sedative hypnotics should not be taken with LUMRYZ
Sedation
Inform patients and/or caregivers that they are likely to fall asleep quickly after taking LUMRYZ (often within 5 and usually within 15 minutes), but the time it takes to fall asleep can vary from night to night. The sudden onset of sleep, including in a standing position or while rising from bed, has led to falls complicated by injuries, in some cases requiring hospitalization
Food Effects on LUMRYZ
Inform patients and/or caregivers that LUMRYZ should be taken at least 2 hours after eating.
Respiratory Depression and Sleep-Disordered Breathing
Inform patients and/or caregivers that LUMRYZ may impair respiratory drive, especially in patients with compromised respiratory function, and may cause apnea
Depression and Suicidality
Instruct patients and/or caregivers to contact a healthcare provider immediately if they develop depressed mood, markedly diminished interest or pleasure in usual activities, significant change in weight and/or appetite, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, or suicidal ideation
Other Behavioral or Psychiatric Adverse Reactions
Inform patients and/or caregivers that LUMRYZ can cause behavioral or psychiatric adverse reactions, including confusion, anxiety, and psychosis. Instruct them to notify their healthcare provider if any of these types of symptoms occur
Sleepwalking
Instruct patients and/or caregivers that LUMRYZ has been associated with sleepwalking and other behaviors during sleep, and to contact their healthcare provider if this occurs
Sodium Intake
Instruct patients and/or caregivers that LUMRYZ contains a significant amount of sodium and patients who are sensitive to sodium intake (e.g., those with heart failure, hypertension, or renal impairment) should limit their sodium intake
Distributed By:
Avadel CNS Pharmaceuticals, LLC
Protected by U.S. Patent No. D1,032,378
7INSTRUCTIONS FOR USE

This Instructions for Use contains information on how to take LUMRYZ. Read this Instructions for Use before you (or your child) take LUMRYZ and each time you (or your child) get a refill. There may be new information.
This information does not take the place of talking to your doctor about your (or your child's) medical condition or your (or your child's) treatment.

● Take (or give) 1 packet of LUMRYZ each day at bedtime.
● You will need to mix LUMRYZ with water before you take or give your child the dose.
● You (or your child) should avoid getting out of bed after taking LUMRYZ. Some people fall asleep within 5 minutes of taking LUMRYZ and most will fall asleep within 15 minutes. The time it takes to fall asleep might be different from night to night.
● Medicines that cause sleepiness should not be used while taking LUMRYZ.
●
●
● Mix and take (or give) LUMRYZ within 30 minutes. If not taken or given within 30 minutes of mixing, throw it away (dispose of it) and prepare a new dose.

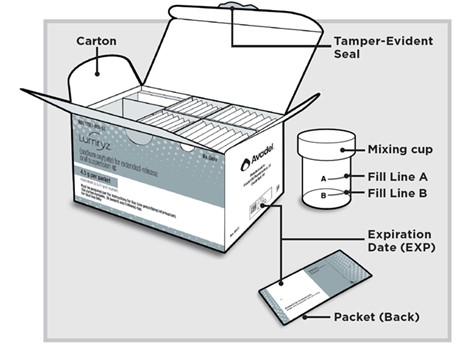
LUMRYZ comes in different package sizes. The LUMRYZ package you receive may look different from the picture shown above.
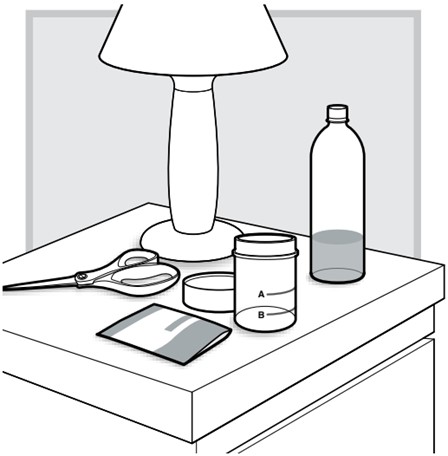
Additional supplies needed
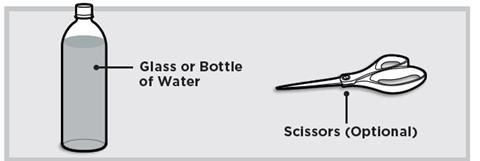

● Store LUMRYZ at room temperature, between 68°F to 77°F (20°C to 25°C).
● Store LUMRYZ in the original packet prior to mixing with water.
● LUMRYZ suspension should be taken within 30 minutes of preparation.
● When you have finished using the LUMRYZ packet, throw it away (dispose of it) in the trash. If any LUMRYZ remains in the packet, rinse it down the sink before throwing away.
● Store LUMRYZ and all medicines out of the reach of children and pets.

● Before using a new LUMRYZ carton, check the tamper-evident seal on the carton lid to make sure it is not missing or broken.
●
● Check the expiration date (EXP) on the LUMRYZ carton.
●
● Open the LUMRYZ carton by tearing the tamper-evident seal with your hands or by using a pair of scissors.

● Clean the mixing cup by rinsing it with water and letting it dry before each use.
●
● Check the expiration date (EXP) on the packet label.
Important: Make sure to prepare LUMRYZ at bedside.
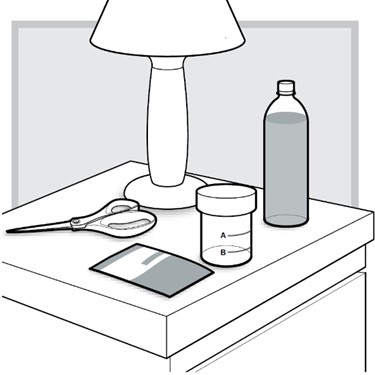

1.) At your (or your child's) bedside, open the mixing cup by twisting the cap to the left (counter-clockwise) to remove it.
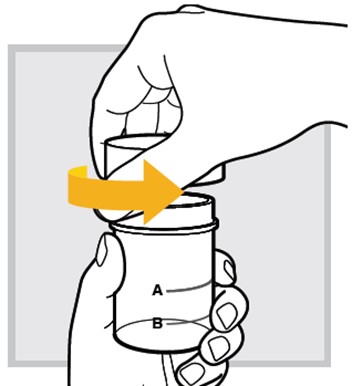
2.) Fill the mixing cup with water up to
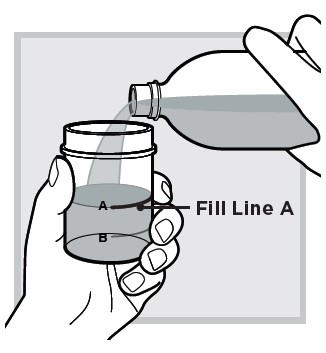
3.) Open 1 packet:
Use scissors to cut open the packet along the
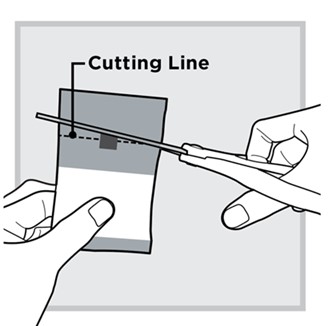
Fold the packet in half at the gray
Tear the packet open with your hands.
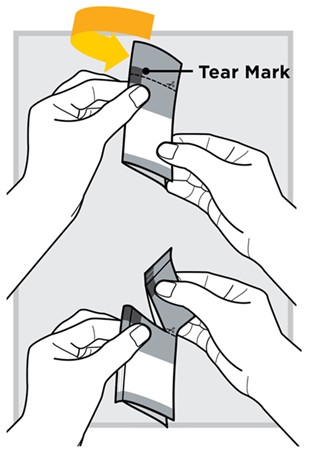
4.) Pour the entire content from the packet into the water-filled mixing cup.
Make sure there is no powder left in the packet.
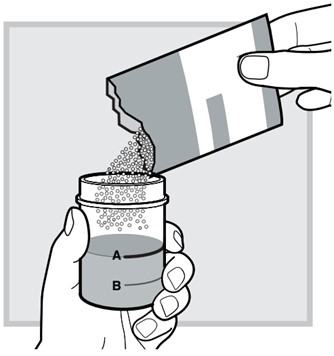
5.) Close the mixing cup by twisting the cap to the right (clockwise) until firmly closed.
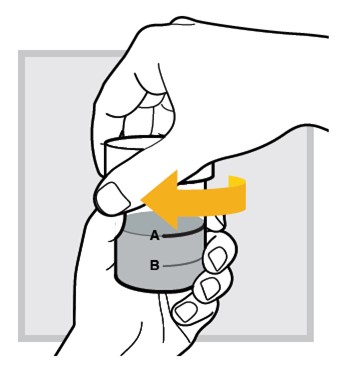
6.) Mix the water and powder solution by shaking the closed mixing cup well for at least
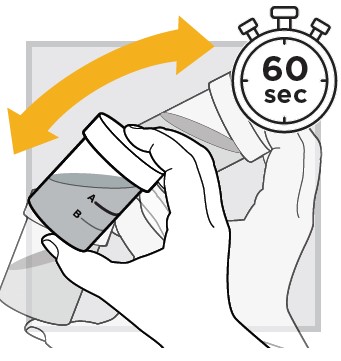
7.) Make sure the solution is mixed thoroughly.
The mixed solution will appear slightly milky with some lumps.
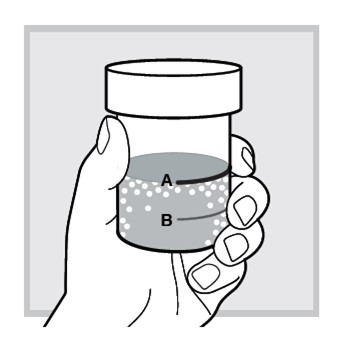
The mixing cup cap is not child resistant. If the mixed solution is not drank immediately, then do not remove the cap, and keep out of reach of children.

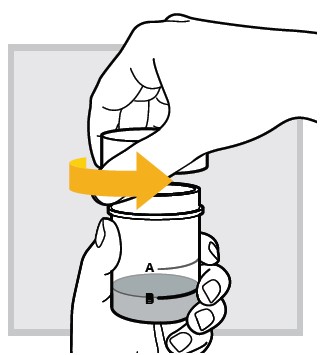
8.) Open the mixing cup by twisting the cap to the left (counter-clockwise) and remove it.
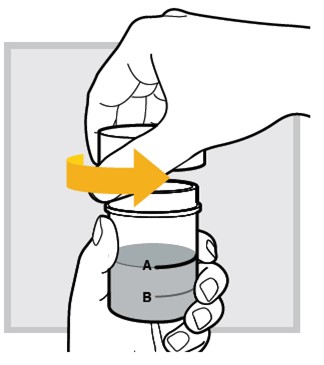
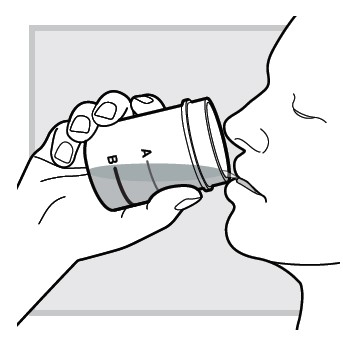
9.) While sitting in bed drink (or have your child drink) the mixed solution within
Make sure you (or your child) drink all the mixed solution in the mixing cup.
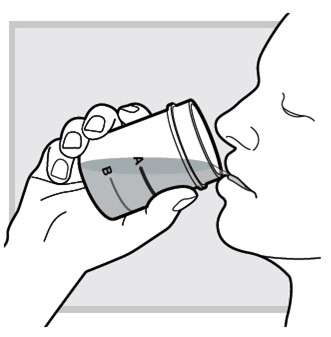
10.) Immediately refill the mixing cup with water up to
Do not open another packet of LUMRYZ. Take (or give) only 1 packet each day at bedtime.
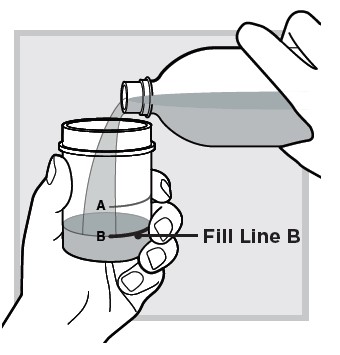
11.) Close the mixing cup by twisting the cap to the right (clockwise) until firmly closed.
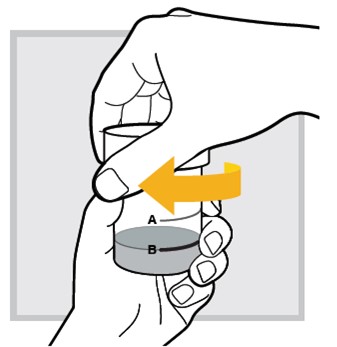
12.) Shake well again for
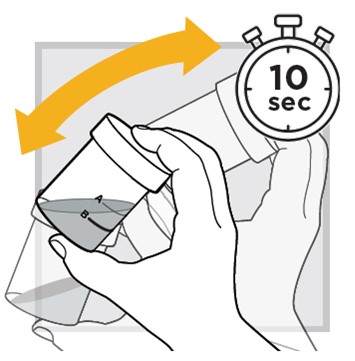
13.) Open the mixing cup by twisting the cap to the left (counter-clockwise) and remove it.
14.) Drink (or have your child drink) the mixed solution immediately after mixing.
Make sure you (or your child) drink all the mixed solution in the mixing cup.
15.) Leave the empty mixing cup at your (or your child's) bedside and immediately lie down (or have your child lie down) to go to sleep.
Avoid getting out of your bed (or having your child get out of bed) after taking LUMRYZ.


16.) The next day, place the empty LUMRYZ packet in the trash.
If any LUMRYZ remains in the packet, rinse it down the sink before (prior to) throwing away (disposal).
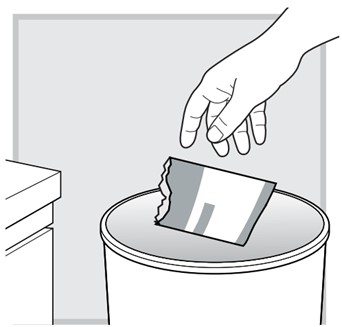
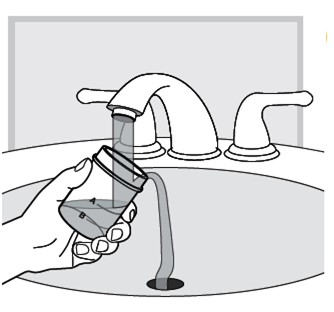
17.) Empty any unused LUMRYZ down the sink drain the next day.
Clean the mixing cup by rinsing it with water and letting it dry before each use.
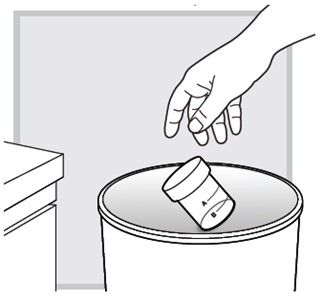
After you (or your child) finish all of the packets in the LUMRYZ carton
After you have (or your child has) finished your (or your child's) last packet in the carton, throw away the rinsed mixing cup in the trash.
If you have additional questions about LUMRYZ, talk with your doctor.
You can also contact:
Avadel CNS Pharmaceuticals, LLC
Chesterfield, MO 63005 USA
For more information on LUMRYZ,
visit
888-8AVADEL (888-828-2335).

© Avadel 2025. All rights reserved. AVADEL, the AVADEL logo, LUMRYZ, and the LUMRYZ logo are trademarks of an Avadel company.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 11/2025
8Principal Display Panel - NDC: 13551-005-01 - Starter Pack Outer Carton Label

