Generic Name
ARIPiprazole
Brand Names
Abilify, Abilify MyCite, Abilify MAINTENA, Abilify Asimtufii, Opipza
FDA approval date: November 15, 2002
Classification: Atypical Antipsychotic
Form: Injection, Tablet, Kit, Film, Solution
What is Abilify (ARIPiprazole)?
Aripiprazole tablets are indicated for the treatment of: Schizophrenia [ see CLINICAL STUDIES ( 1.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ABILIFY (ARIPIPRAZOLE)
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and SUICIDAL THOUGHTS AND BEHAVIORS WITH ANTIDEPRESSANT DRUGS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. ABILIFY is not approved for the treatment of patients with dementia-related psychosis
Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients over age 24 years; there was a reduction in risk with antidepressant use in patients aged 65 years and older
In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber
1INDICATIONS AND USAGE
ABILIFY (aripiprazole) Tablets are indicated for the treatment of:
- Schizophrenia
- Acute Treatment of Manic and Mixed Episodes associated with Bipolar I Disorder
- Adjunctive Treatment of Major Depressive Disorder
- Irritability Associated with Autistic Disorder
- Treatment of Tourette's Disorder
2DOSAGE FORMS AND STRENGTHS
ABILIFY
3CONTRAINDICATIONS
ABILIFY is contraindicated in patients with a history of a hypersensitivity reaction to aripiprazole. Reactions have ranged from pruritus/urticaria to anaphylaxis
4ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis
- Cerebrovascular Adverse Events, Including Stroke
- Suicidal Thoughts and Behaviors in Children, Adolescents, and Young Adults
- Neuroleptic Malignant Syndrome (NMS)
- Tardive Dyskinesia
- Metabolic Changes
- Pathological Gambling and Other Compulsive Behaviors
- Orthostatic Hypotension
- Falls
- Leukopenia, Neutropenia, and Agranulocytosis
- Seizures/Convulsions
- Potential for Cognitive and Motor Impairment
- Body Temperature Regulation
- Suicide
- Dysphagia
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions in adult patients in clinical trials (≥10%) were nausea, vomiting, constipation, headache, dizziness, akathisia, anxiety, insomnia, and restlessness.
The most common adverse reactions in the pediatric clinical trials (≥10%) were somnolence, headache, vomiting, extrapyramidal disorder, fatigue, increased appetite, insomnia, nausea, nasopharyngitis, and weight increased.
ABILIFY has been evaluated for safety in 13,543 adult patients who participated in multiple-dose, clinical trials in schizophrenia, bipolar disorder, major depressive disorder, dementia of the Alzheimer's type, Parkinson's disease, and alcoholism, and who had approximately 7,619 patient-years of exposure to oral ABILIFY and 749 patients with exposure to aripiprazole injection. A total of 3,390 patients were treated with oral ABILIFY for at least 180 days and 1,933 patients treated with oral ABILIFY had at least one year of exposure.
ABILIFY has been evaluated for safety in 1,686 pediatric patients (6 to 18 years) who participated in multiple-dose, clinical trials in schizophrenia, bipolar mania, autistic disorder, or Tourette's Disorder and who had approximately 1,342 patient-years of exposure to oral ABILIFY. A total of 959 pediatric patients were treated with oral ABILIFY for at least 180 days and 556 pediatric patients treated with oral ABILIFY had at least one year of exposure.
The conditions and duration of treatment with ABILIFY (monotherapy and adjunctive therapy with antidepressants or mood stabilizers) included (in overlapping categories) double-blind, comparative and noncomparative open-label studies, inpatient and outpatient studies, fixed- and flexible-dose studies, and short- and longer-term exposure.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ABILIFY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: occurrences of allergic reaction (anaphylactic reaction, angioedema, laryngospasm, pruritus/urticaria, or oropharyngeal spasm), blood glucose fluctuation, drug reaction with eosinophilia and systemic symptoms (DRESS), hiccups, oculogyric crisis, pathological gambling, and fecal incontinence.
5OVERDOSAGE
MedDRA terminology has been used to classify the adverse reactions.
6DESCRIPTION
Aripiprazole is an atypical antipsychotic drug that is available as ABILIFY
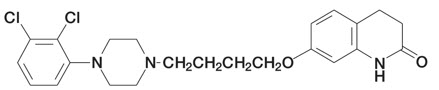
ABILIFY Tablets are available in 2 mg, 5 mg, 10 mg, 15 mg, 20 mg, and 30 mg strengths. Inactive ingredients include cornstarch, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. Colorants include ferric oxide (yellow or red) and FD&C Blue No. 2 Aluminum Lake.
7CLINICAL STUDIES
Efficacy of ABILIFY was established in the following adequate and well-controlled trials:
- Four short-term trials and one maintenance trial in adult patients and one short-term trial in adolescents (ages 13 to 17 years) with schizophrenia
- Four short-term monotherapy trials and one 6-week adjunctive trial in adult patients and one short-term monotherapy trial in pediatric patients (ages 10 to 17 years) with manic or mixed episodes
- One maintenance monotherapy trial and one maintenance adjunctive trial in adult patients with bipolar I disorder
- Two short-term trials in adult patients with MDD who had an inadequate response to antidepressant therapy during the current episode
- Two short-term trials in pediatric patients (ages 6 to 17 years) for the treatment of irritability associated with autistic disorder
- Two short-term trials in pediatric patients (ages 6 to 18 years) with Tourette's Disorder
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
9PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
30 Tablets NDC 59148-006-13
10PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
30 Tablets NDC 59148-007-13

11PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
30 Tablets NDC 59148-008-13

12PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
30 Tablets NDC 59148-009-13

13PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
30 Tablets NDC 59148-010-13

14PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
30 Tablets NDC 59148-011-13
