Steglatro
What is Steglatro (Ertugliflozin)?
Steglatro (Ertugliflozin): An SGLT2 Inhibitor for Type 2 Diabetes
Living with type 2 diabetes means managing a condition that affects nearly every part of your day, from what you eat to how you feel. It can be a constant balancing act, and finding the right tools to help you manage your blood sugar is a critical part of staying healthy and feeling your best. When diet and exercise alone aren’t enough to reach your goals, your doctor may prescribe medication to help.
Steglatro (ertugliflozin) is a prescription medicine used to help control blood sugar levels in adults with type 2 diabetes. It is not for type 1 diabetes. Steglatro belongs to a class of drugs known as **sodium-glucose co-transporter 2 (SGLT2) inhibitors**. It is an oral medication that works alongside your diet and exercise plan to help lower your blood sugar and improve your A1C.
What does Steglatro do?
Steglatro is approved by the U.S. Food and Drug Administration (FDA) to improve glycemic control in adults with type 2 diabetes, in addition to diet and exercise. This means its primary job is to help lower high blood sugar (**hyperglycemia**) (FDA, 2024).
By helping to keep your blood sugar levels in a healthier range, Steglatro can be an important part of your diabetes management plan. Effectively managing blood sugar is key to preventing or delaying the serious long-term complications of diabetes, such as nerve damage, kidney problems, vision loss, and heart disease. While Steglatro helps lower blood sugar, it is **not approved to reduce the risk of heart attack or stroke**.
How does Steglatro work?
The mechanism of Steglatro is unique and does not depend on your body’s insulin production. Instead, it works directly with your kidneys.
Your kidneys act as your body’s filters. As blood passes through them, they filter out waste products while also reabsorbing essential substances back into the bloodstream, including sugar (glucose). In people with type 2 diabetes, the body often reabsorbs too much sugar, even when blood sugar levels are already high.
Steglatro works by blocking a specific protein in the kidneys called **SGLT2**. This SGLT2 protein is the main pathway responsible for pulling sugar back into the body. By inhibiting SGLT2, Steglatro prevents the kidneys from reabsorbing excess sugar. As a result, that extra sugar is **removed from your body through your urine** (U.S. National Library of Medicine, 2022).
Steglatro Side Effects and Warnings
Like any medication, Steglatro can cause side effects. Because it works by removing sugar through your urine, the most common side effects are related to the genital and urinary tracts. These include:
Common Side Effects:
- Female genital yeast infections
- Male yeast infections (balanitis or balanoposthitis)
- Urinary tract infections (UTIs)
- Increased urination or urinating more often
It is important to practice good hygiene and drink plenty of fluids to help prevent these. Contact your doctor if you experience symptoms like itching, unusual discharge, pain or burning when urinating, or a frequent urge to go.
Serious Side Effect Warnings (Seek immediate medical help or call your doctor for):
- Ketoacidosis: High blood acids (ketones) causing nausea, vomiting, stomach pain, fatigue, and trouble breathing, even with normal blood sugar.
- Serious UTIs: Infections potentially spreading to kidneys or bloodstream.
- Fournier’s Gangrene: Rare, life-threatening bacterial infection of the perineum. Seek emergency care for tenderness, redness, swelling, fever, or feeling unwell in that area.
- Lower Limb Amputation: Increased risk, mainly of the toe. Daily foot checks for pain, sores, ulcers, or infections are crucial; report immediately.
- Dehydration (Volume Depletion): Increased urination can lead to dizziness, fainting, or weakness, especially when standing (orthostatic hypotension) (FDA, 2024).
Steglatro is not for everyone. You should not take it if you have **severe kidney problems** or are on dialysis, or if you have had a serious allergic reaction to it.
Steglatro Dosage and Monitoring
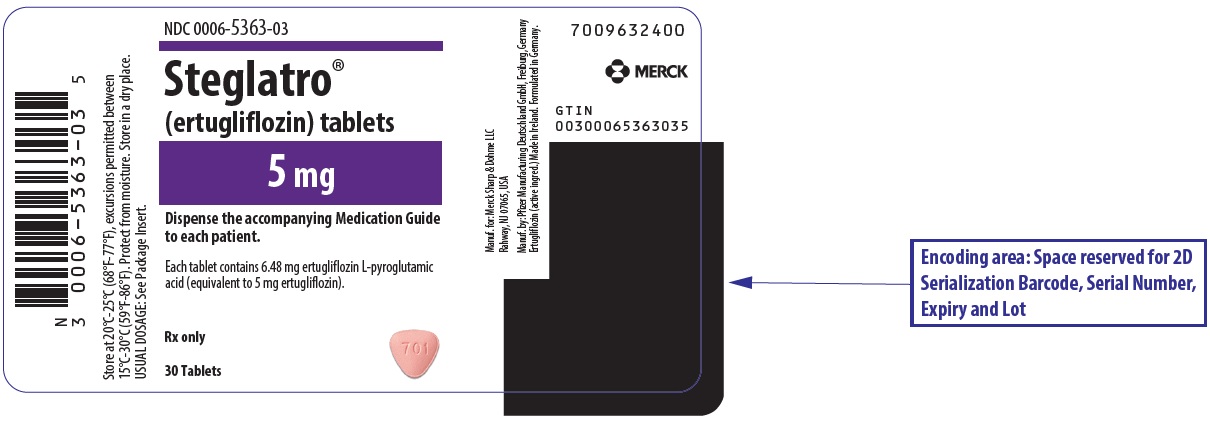
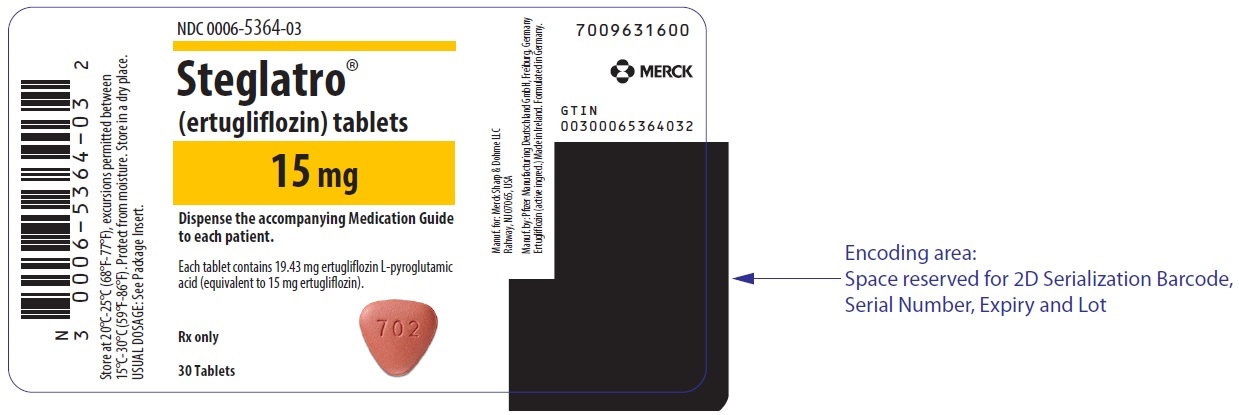
Steglatro comes as an oral tablet, available in 5 mg and 15 mg strengths. It is typically taken **once per day, in the morning, with or without food.**
The starting dose is usually 5 mg once daily. Your doctor may increase your dose to 15 mg once daily if you tolerate the medication well but need additional blood sugar control (FDA, 2024). The specific dose prescribed for you depends on your medical history, kidney function, and other medications you may be taking.
Before starting Steglatro, your doctor will check your kidney function (**eGFR**) and monitor it periodically. Steglatro is not recommended for severely reduced kidney function. Take as prescribed.
Does Steglatro have a generic version?
No, Steglatro does not currently have a generic version available in the United States. It is a brand-name medication, and its active ingredient is **ertugliflozin**. However, international versions may exist in other markets.
When a drug is first approved by the FDA, it is protected by patents that prevent other companies from manufacturing generic versions for a certain period. Once these patents expire, other manufacturers can apply to the FDA to sell generic versions. Generics must be proven to be just as safe and effective as the brand-name drug, offering the same active ingredient, strength, and dosage form (FDA, 2023).
Conclusion
Steglatro is an effective once-daily medication that offers a unique way to help manage type 2 diabetes. By working with your kidneys to remove excess sugar from your body, it can be a valuable tool, alongside diet and exercise, to help you reach your blood sugar targets.
Steglatro offers benefits but carries risks like genital infections, UTIs, and rarely, more serious issues. Safe use requires close partnership with your doctor, including regular check-ups, kidney function monitoring, and diligent foot care. Stay informed, take medication as prescribed, and discuss any concerns with your doctor.
References
- U.S. Food and Drug Administration (FDA). (2023). Generic drug facts. Retrieved from fda.gov/drugs/generic-drugs/generic-drug-facts
- U.S. Food and Drug Administration (FDA). (2024). Steglatro (ertugliflozin) label. Retrieved from accessdata.fda.gov/drugsatfda_docs/label/2024/209803s022lbl.pdf
- U.S. National Library of Medicine. (2022). Ertugliflozin. MedlinePlus. Retrieved from medlineplus.gov/druginfo/meds/a617044.html
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to determine the effects on heart failure signs and symptoms of the use of either ertugliflozin, metolazone or placebo, in conjunction with intravenous loop diuretic use in acute settings and chronic oral loop diuretic therapy. There are two general purposes for this study. The proposed study is both larger and more rigorous than essentially all PK/PD studies that form...
Summary: SGLT2 inhibitors have demonstrated to mitigate cardiorenal risk in people with type 2 diabetes and are likely to play an increasingly large role in the treatment of patients with diabetes, chronic kidney disease and hypertension. Yet the underlying mechanisms of its protective effects are incompletely understood and the salutary effect may be altered by dietary factors such as sodium intake. There...
Summary: Open-label, prospective, single-arm, multicenter study to determine effects of Ertugliflozin on liver fat, liver fibrosis \& glycemic control in subjects with Type 2 Diabetes Mellitus (T2DM) with Non-Alcoholic Fatty Liver Disease (NAFLD)/Non-Alcoholic Steatohepatitis (NASH)
Related Latest Advances
Brand Information
- Tablets: 5 mg, pink, triangular-shaped debossed with "701" on one side and plain on the other side.
- Tablets: 15 mg, red, triangular-shaped debossed with "702" on one side and plain on the other side.
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes and Other Ketoacidosis
- Lower Limb Amputation
- Volume Depletion
- Urosepsis and Pyelonephritis
- Hypoglycemia with Concomitant Use with Insulin or Insulin Secretagogues
- Necrotizing Fasciitis of the Perineum (Fournier's Gangrene)
- Genital Mycotic Infections
- Infections: necrotizing fasciitis of the perineum (Fournier's Gangrene)
- Skin and Subcutaneous Tissue Disorders: angioedema, rash