Candesartan Cilexetil
What is Atacand (Candesartan Cilexetil)?
Approved To Treat
Related Clinical Trials
Summary: Changes to gastric pH, gastric emptying time, gastrointestinal transit-time or the pre-systemic metabolizing effect of enzymes secreted in the mucosa may all alter the pharmacokinetics of medicines. These factors are potentially influenced by bariatric surgery. Little is so far known about how gastric bypass and sleeve gastrectomy impacts the biological availability of medication. In this study th...
Objective: This study aims to evaluate the safety and efficacy of the fixed-dose triple combination therapy (SPC1001) of candesartan, amlodipine, and indapamide in adult patients with essential hypertension compared to dual-component therapies of each ingredient for 8 weeks. Additionally, it seeks to confirm the contribution of each component at low doses. \- Inclusion Criteria: Men and women aged 19 to 75 y...
Summary: The purpose of this study is to investigate the effect of sacubitril/valsartan on cardiac function assessed by cardiac magnetic resonance (CMR) in hypertensive patients stratified by BMI.
Related Latest Advances
Brand Information
- When pregnancy is detected, discontinue ATACAND as soon as possible
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus
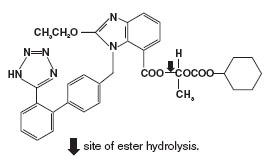
- treat high blood pressure in adults and children, 1 to 17 years of age
- treat certain types of heart failure in adults, to reduce death and hospitalization for heart damage and heart failure
- are allergic to any of the ingredients in ATACAND. See the end of this leaflet for a complete list of ingredients in ATACAND.
- are diabetic and taking aliskiren.
- have heart problems
- have liver problems
- have kidney problems
- currently have vomiting or diarrhea
- are scheduled for surgery or anesthesia. Low blood pressure can happen in people who take ATACAND and have major surgery and anesthesia.
- have any other medical conditions
- are pregnant or planning to become pregnant. See “What is the most important information I should know about ATACAND?”
- are breast-feeding or plan to breast-feed. It is not known if ATACAND passes into your breast milk. You and your doctor should decide if you will take ATACAND or breast-feed. You should not do both.
- lithium carbonate (Lithobid) or lithium citrate, medicines used in some types of depression
- other medicines for high blood pressure, especially water pills (diuretics)
- potassium supplements
- salt substitutes
- non-steroidal anti-inflammatory drugs (NSAIDs)
- Take ATACAND exactly as prescribed by your doctor.
- Do not change your dose or stop ATACAND without talking to your doctor, even if you are feeling well.
- If your child cannot swallow tablets, or if tablets are not available in the prescribed strength, your pharmacist will prepare ATACAND as a liquid suspension for your child. If your child switches between taking the tablet and the suspension, your doctor will change the dose as needed. Shake the bottle of suspension well before each dose.
- ATACAND is taken by mouth with or without food.
- If you miss a dose of ATACAND, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Take the next dose on time. Do not take 2 doses at one time. If you are not sure about your dosing call your doctor or pharmacist.
- If you take more ATACAND than prescribed, call your doctor, local poison control center, or go to the nearest emergency room.
- Injury or death to your unborn baby. See “What is the most important information I should know about ATACAND?”
- Low blood pressure (hypotension). Low blood pressure is most likely to happen if you:
- take water pills (diuretics)
- are on a low salt diet
- get dialysis treatments
- are dehydrated (decreased body fluids) due to vomiting and diarrhea
- have heart problems
- Worsening kidney problems. Kidney problems may get worse in people that already have kidney disease or heart problems. Your doctor may do blood tests to check for this.
- Increased potassium in your blood. Your doctor may do a blood test to check your potassium levels as needed.
- Symptoms of allergic reaction. Call your doctor right away if you have any of these symptoms of an allergic reaction:
- swelling of your face, lips, tongue or throat
- rash
- hives and itching
- back pain
- dizziness
- cold or flu symptoms (upper respiratory tract infection)
- sore throat (pharyngitis)
- nasal congestion and stuffiness (rhinitis)
- Do not keep medicine that is out of date or that you no longer need.
- Store ATACAND tablets at room temperature below 86°F (30°C).
- Store ATACAND oral suspension at room temperature below 86°F (30°C).
- Use the oral suspension within 30 days after first opening the bottle. Do not use after the expiration date stated on the bottle.
- Do not freeze.
- Keep the container of ATACAND closed tightly.








