Adempas
What is Adempas (Riociguat)?
Approved To Treat
Related Clinical Trials
Summary: The main goal of this study is to determine the effects of combination medical therapy (Riociguat and Macitentan) and balloon pulmonary angioplasty (BPA) on hemodynamics and right ventricular (RV) function (including advanced assessments of RV-pulmonary artery (PA) coupling from invasive hemodynamics) in participants with inoperable or post-PTE residual CTEPH.
Summary: This study was designed to investigate the safety and efficacy of replacing phosphodiesterase 5 inhibitors (PDE5i) with riociguat in patients with Chronic thromboembolic pulmonary hypertension (CTEPH) who have undergone pulmonary angioplasty (BPA) and remains symptomatic despite treatments with PDE5i.
Summary: Riociguat and balloon pulmonary angioplasty (BPA) are established standard-of-care interventions for inoperable chronic thromboembolic pulmonary hypertension (CTEPH) with comparable evidence levels. However, the optimal combined treatment strategy remains unclear. Specifically, there is no consensus on whether riociguat should be continued long-term after achieving hemodynamic stability with BPA. ...
Related Latest Advances
Brand Information
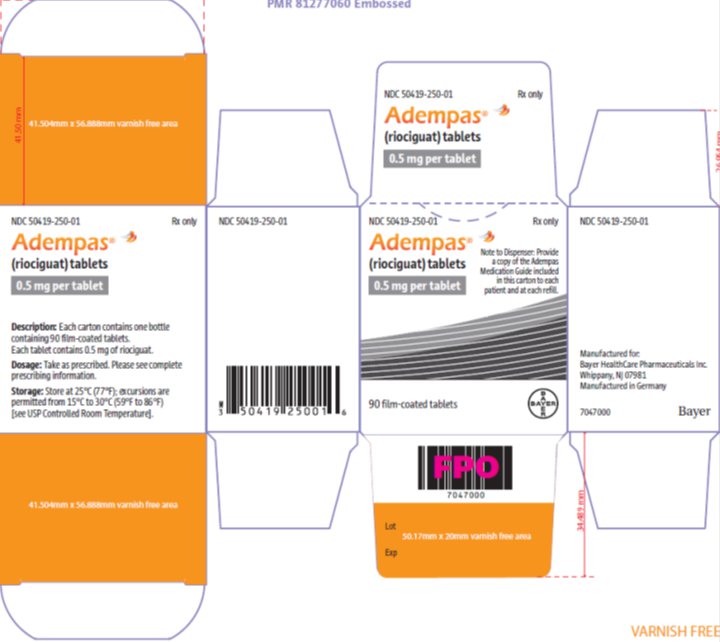
- 0.5 mg, white, with “BAYER” cross on one side and “0.5” and “R” on the other side
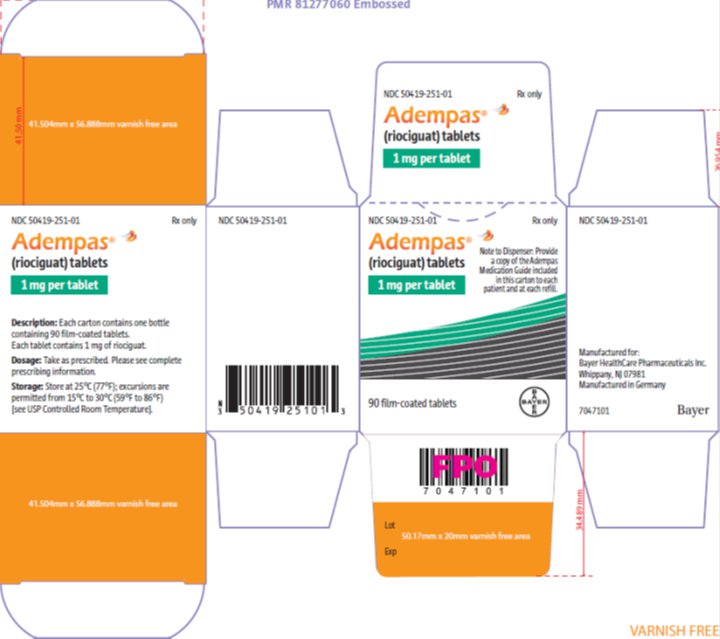
- 1 mg, pale-yellow, with “BAYER” cross on one side and “1” and “R” on the other side
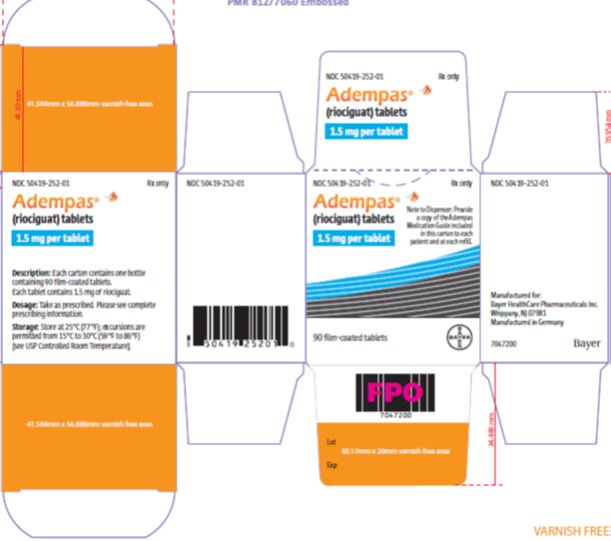
- 1.5 mg, yellow-orange, with “BAYER” cross on one side and “1.5” and “R” on the other side
- 2 mg, pale orange, with “BAYER” cross on one side and “2” and “R” on the other side
- 2.5 mg, red-orange, with “BAYER” cross on one side and “2.5” and “R” on the other side
- Embryo-Fetal Toxicity
- Hypotension
- Bleeding
- All female patients must sign an enrollment form.
- Advise female patients of reproductive potential that she must comply with the pregnancy testing and contraception requirements
- Educate and counsel females of reproductive potential on the use of emergency contraception in the event of unprotected sex or contraceptive failure.
- Advise pre-pubertal females to report any changes in their reproductive status immediately to her prescriber.
- Inform patients of the contraindication of Adempas with nitrates or nitric oxide donors or PDE-5 inhibitors.
- Advise patients about the potential risks/signs of hemoptysis and to report any potential signs of hemoptysis to their physicians.
- Instruct patients on the dosing, titration, and maintenance of Adempas.
- Advise patients regarding activities that may impact the pharmacology of Adempas (strong multi pathway CYP inhibitors and P-gp/BCRP inhibitors and smoking). Instruct patients to report all current medications and new medications to their physician.
- Advise patients that antacids should not be taken within 1 hour of taking Adempas.
- Inform patients that Adempas can cause dizziness, which can affect the ability to drive and use machines
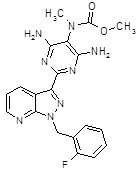
(riociguat)
- Serious birth defects.
- Adempas can cause serious birth defects if taken during pregnancy.
- Females must not be pregnant when they start taking Adempas or become pregnant during treatment with Adempas.
- Females who are able to get pregnant must have a negative pregnancy test before beginning treatment with Adempas, each month during treatment, and 1 month after you stop treatment with Adempas. Talk to your doctor about your menstrual cycle. Your doctor will decide when to do the tests, and order the tests for you depending on your menstrual cycle.
- Have entered puberty, even if they have not started their period, and
- Have a uterus, and
- If you have had a tubal sterilization, have a progesterone implant, or have an IUD (intrauterine device), these methods can be used alone and no other form of birth control is needed.
- If you decide that you want to change the form of birth control that you use, talk with your doctor or gynecologist to be sure that you choose another acceptable form of birth control.

- Do not have unprotected sex. Talk to your doctor or pharmacist right away if you have unprotected sex or if you think your birth control has failed. Your doctor may talk with you about using emergency birth control.
- Tell your doctor right away if you miss a menstrual period or think you may be pregnant for any reason.
- chronic thromboembolic pulmonary hypertension (CTEPH)
- CTEPH is a type of high blood pressure in the arteries of your lungs caused by blood clots that narrow or block blood flow. Adempas can improve your ability to exercise and can help to improve some of your symptoms.
- pulmonary arterial hypertension (PAH)
- PAH is a type of high blood pressure in the arteries of your lungs. Adempas can improve your ability to exercise, improve some of your symptoms, and help slow down the worsening of your physical condition.
- It is unknown if Adempas is safe and effective in children.
- you are pregnant, plan to become pregnant, or become pregnant during treatment with Adempas. Adempas can cause serious birth defects. (See the Medication Guide section above called "What is the most important information I should know about Adempas?")
- you take:
- another medicine called a soluble guanylate cyclase stimulator (sGC). Ask your healthcare provider if you are not sure if you are taking an sGC medicine.
- a nitrate medicine to treat high blood pressure or heart disease, such as nitroglycerin, or a medicine called a nitric oxide donor, such as amyl nitrite
- certain other medicines that contain sildenafil (Revatio or Viagra), tadalafil (Adcirca or Cialis), vardenafil (Levitra or Staxyn), dipyridamole, or theophylline. Revatio and Adcirca are also used to treat PAH
- you have pulmonary hypertension associated with idiopathic interstitial pneumonias (PH-IIP).
- smoke
- have recently had serious bleeding from your lung, or if you have had a medical procedure called bronchial arterial embolization to stop you from coughing up blood
- have problems with your heart or blood circulation
- have low blood pressure
- have liver problems
- have kidney problems or are on dialysis
- have narrowing of the pulmonary veins, a condition called pulmonary veno-occlusive disease or PVOD
- have any other medical conditions
- Do not take Adempas within 24 hours of sildenafil. Do not take Adempas 24 hours before or within 48 hours after tadalafil.
- Take Adempas exactly as your doctor tells you. Do not stop taking Adempas or change your dose without talking to your doctor.
- When you begin treatment with Adempas, your blood pressure should be monitored about every 2 weeks to help your doctor decide the correct dose of medicine for you.
- Your doctor may change your dose during treatment, especially when you first start taking Adempas. It is important to tell your doctor if you have any symptoms of low blood pressure during this time, such as dizziness, lightheadedness, or fainting.
- Take Adempas 3 times each day, about 6 to 8 hours apart.
- Take Adempas with or without food.
- Do not take more than a total of 7.5 mg of Adempas in 1 day unless your doctor tells you to.
- If you take a heartburn medicine (antacid) that contains aluminum hydroxide or magnesium hydroxide, do not take it within
- If you take too much Adempas, call your doctor right away or go to the nearest hospital emergency room.
- If you miss a dose, take your next dose of Adempas at the regular time.
- If you miss 3 or more days of treatment with Adempas, call your doctor for instructions before you restart Adempas.
- Do not get pregnant while taking Adempas. (See serious birth defects section of the Medication Guide above called "What is the most important information I should know about Adempas?") If you miss a menstrual period, or think you might be pregnant, call your doctor right away.
- It is not known if Adempas passes into your breast milk. You should not breastfeed if you take Adempas. Talk to your doctor about the best way to feed your baby if you take Adempas.
- Adempas may make you feel dizzy.
- Smoking. Adempas may not work as well if you smoke during treatment. Tell your doctor if you stop smoking or start smoking during treatment with Adempas, because your dose of Adempas may need to be changed.
- Serious birth defects. (See "What is the most important information I should know about Adempas?")
- Reduced blood pressure. Adempas reduces blood pressure. This may cause symptoms of low blood pressure, such as lightheadedness, chest pain, and dizziness especially in people who are dehydrated, or have a severe blockage of blood flow out of the heart, or have certain other medical problems. Your doctor will check you for these problems.
- Increased risk of bleeding, including bleeding from the respiratory tract. Tell your doctor right away if you cough up blood during treatment with Adempas.
- Worsening of symptoms in people with Pulmonary Veno-Occlusive Disease (PVOD). If you have PVOD, treatment with Adempas may cause a build-up of fluid in your lungs (pulmonary edema). This may cause you to feel short of breath. Your doctor may tell you to stop taking Adempas and switch you to a different medicine.
- The most common side effects of Adempas are:
- headache
- dizziness
- indigestion
- swelling of your hands, legs, feet, and ankles (peripheral edema)
- nausea, diarrhea, and vomiting
- Store Adempas at room temperature between 59° F to 86° F (15° C to 30° C)