Temodar
What is Temodar (Temozolomide)?
Approved To Treat
Related Clinical Trials
Background: Diffuse midline gliomas are the most aggressive brain tumors of childhood and young adults. Most people with these tumors survive less than 2 years. Researchers want to see if an anticancer drug (abemaciclib) can help.
Summary: This is a 2-part study. The purpose of Part 1 of the study is to evaluate the efficacy, safety, and pharmacokinetic (PK) characteristics of safusidenib in participants with recurrent/progressive IDH1-mutant World Health Organization (WHO) Grade 2 or Grade 3 glioma. The purpose of Part 2 will be to evaluate the efficacy of maintenance safusidenib treatment versus placebo in IDH1-mutant Grade 3 astr...
Summary: This study will test the safety and effectiveness of a combination of pembrolizumab, olaparib, and temozolomide to see how well these drugs work when given together in people with a glioma that either did not respond to previous treatment or came back after treatment.
Related Latest Advances
Brand Information
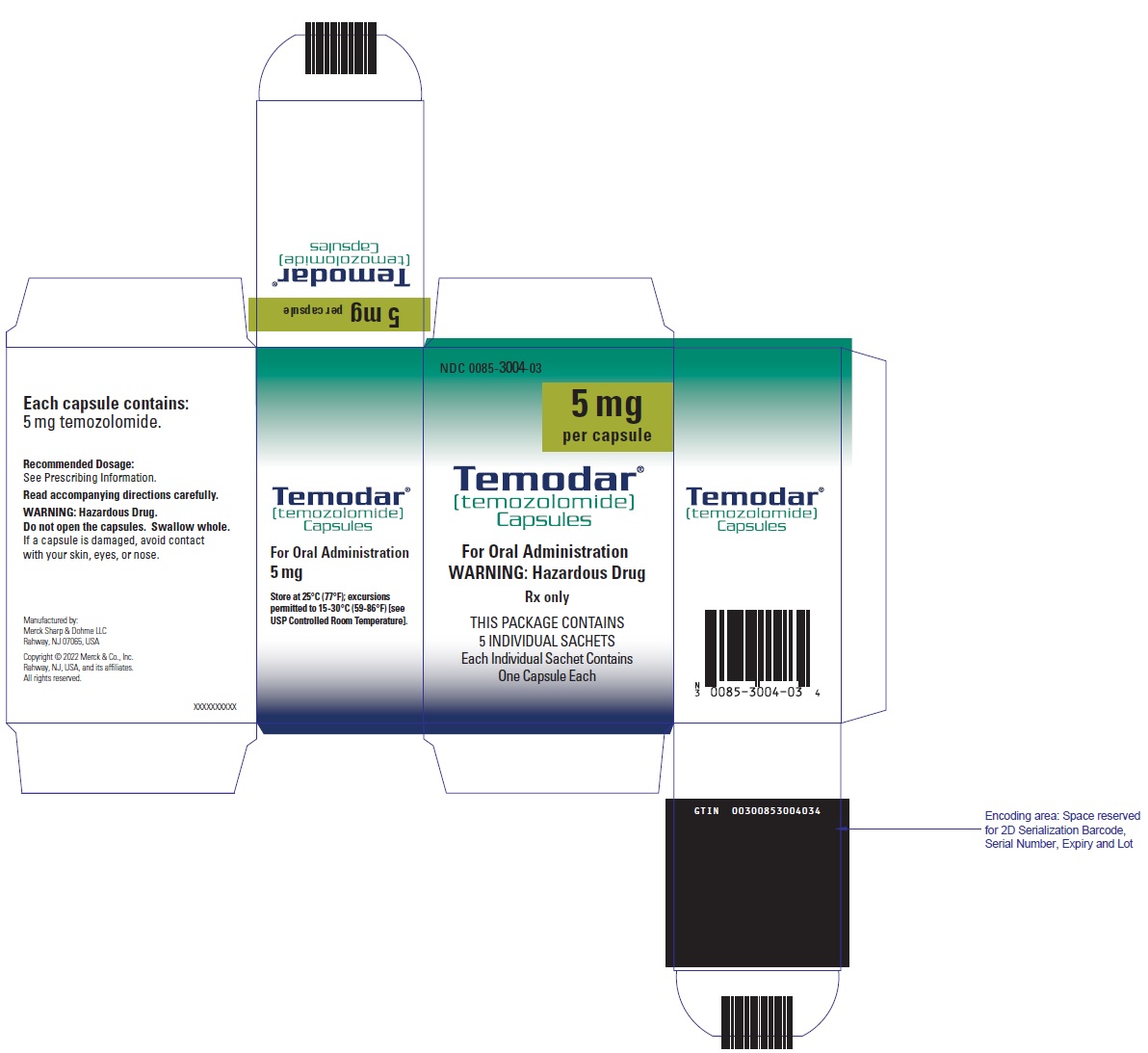
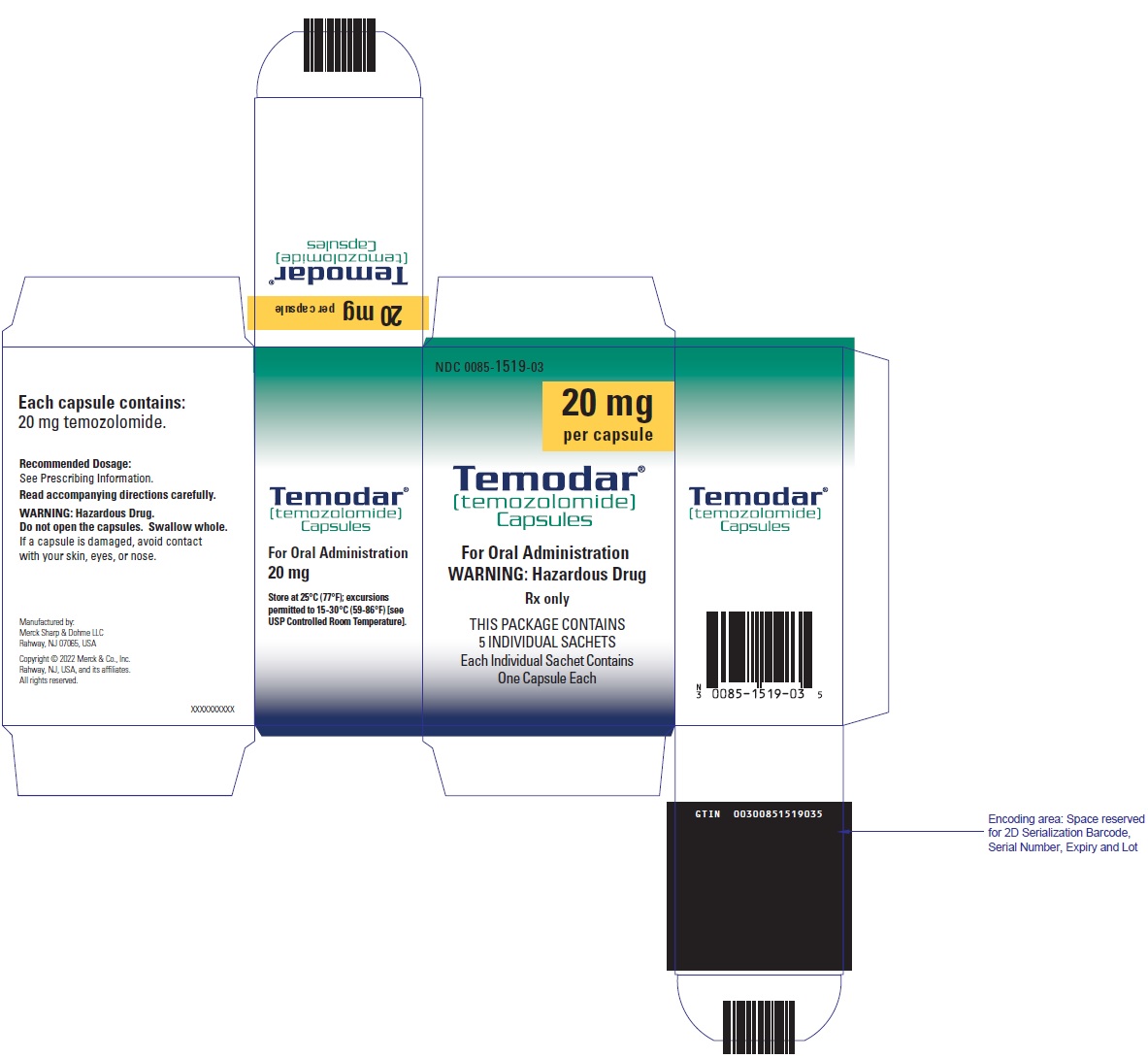
- Capsules:
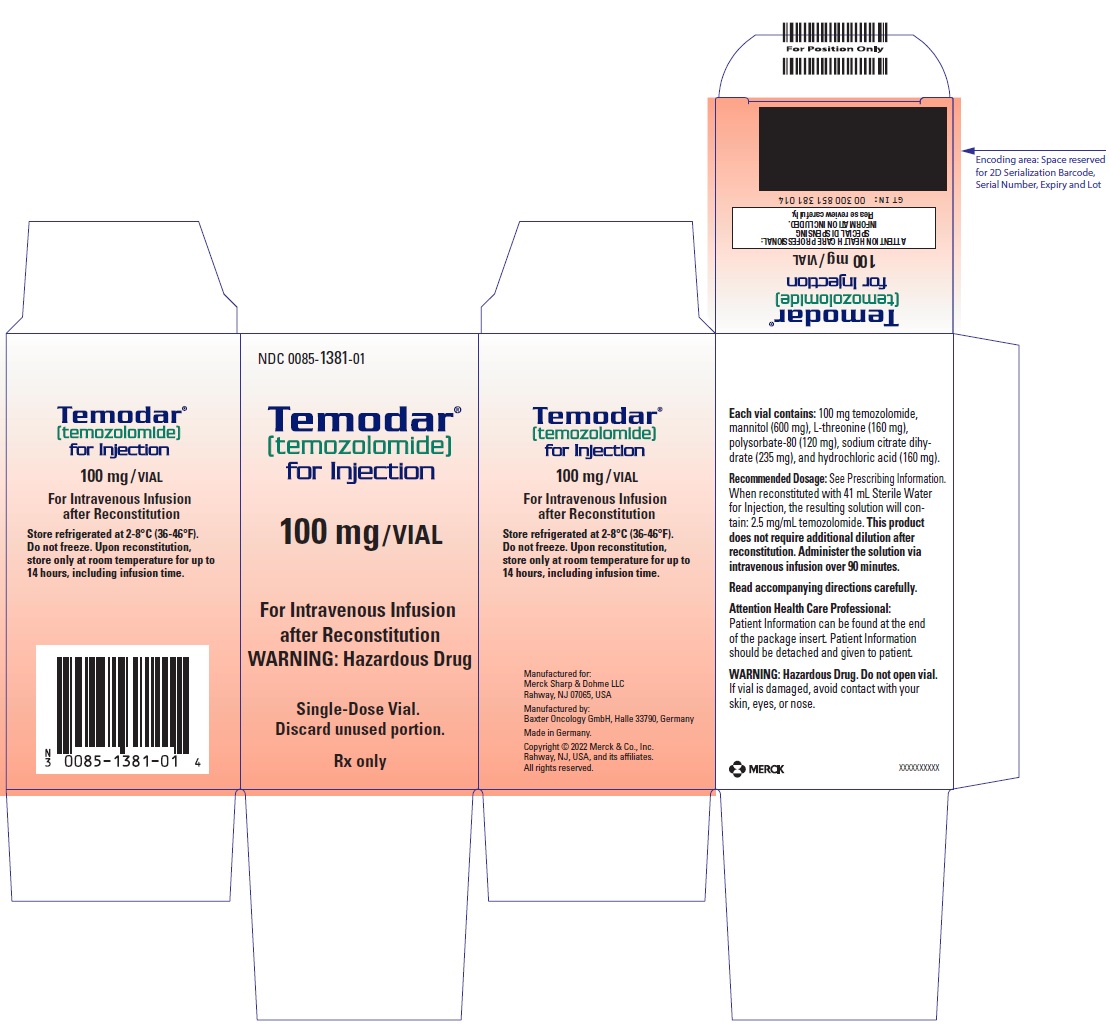
- For injection: 100 mg white to light tan or light pink lyophilized powder for reconstitution in a single-dose vial.
- temozolomide or any other ingredients in TEMODAR; and
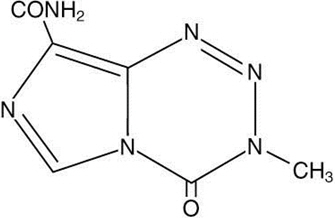
- dacarbazine, since both temozolomide and dacarbazine are metabolized to the same active metabolite 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide.
- Myelosuppression
- Hepatotoxicity
- Pneumocystis Pneumonia [see
- Secondary Malignancies
- TEMODAR 5 mg: lactose anhydrous (132.8 mg), colloidal silicon dioxide (0.2 mg), sodium starch glycolate (7.5 mg), tartaric acid (1.5 mg), and stearic acid (3 mg).
- TEMODAR 20 mg: lactose anhydrous (182.2 mg), colloidal silicon dioxide (0.2 mg), sodium starch glycolate (11 mg), tartaric acid (2.2 mg), and stearic acid (4.4 mg).
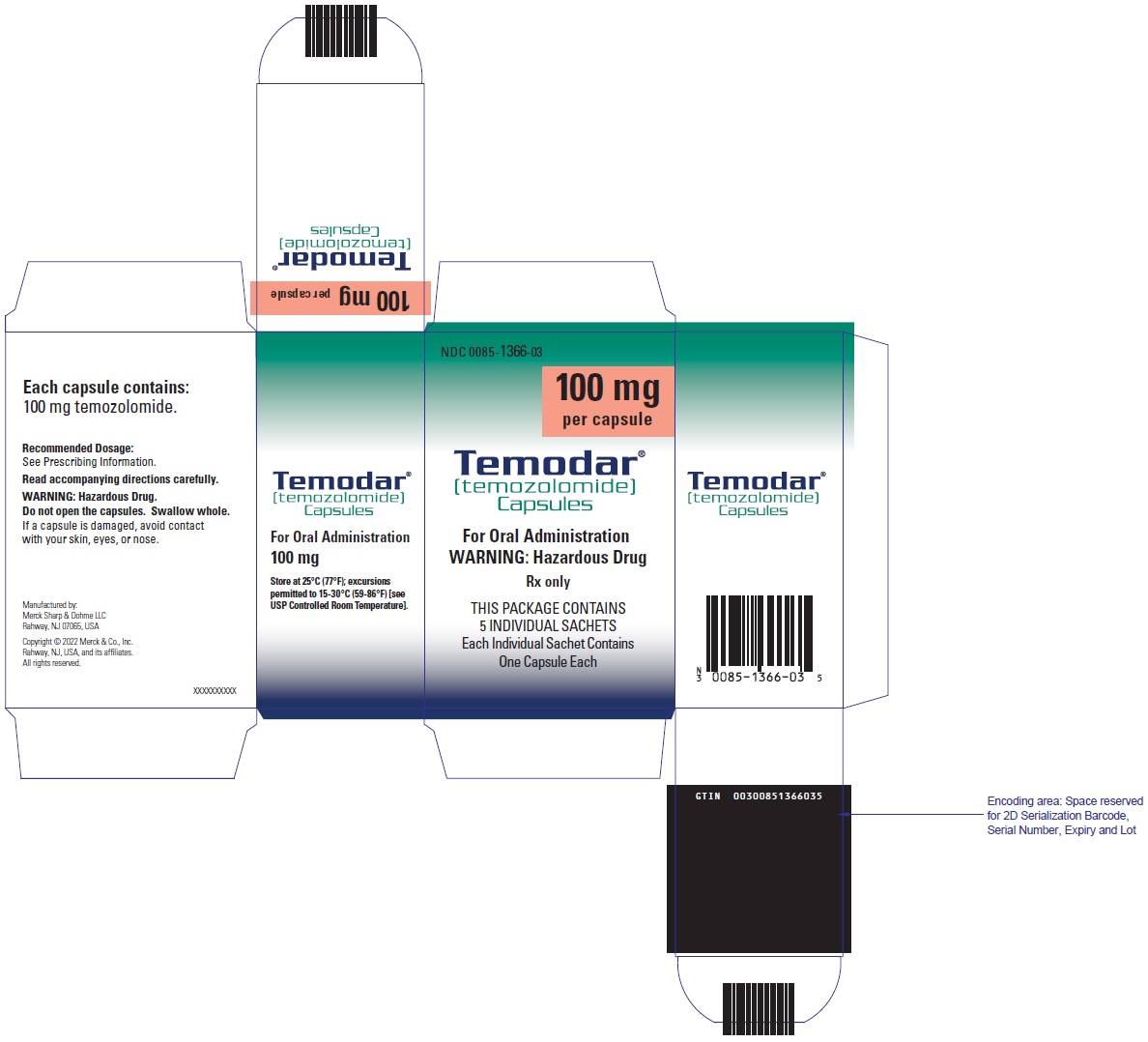
- TEMODAR 100 mg: lactose anhydrous (175.7 mg), colloidal silicon dioxide (0.3 mg), sodium starch glycolate (15 mg), tartaric acid (3 mg), and stearic acid (6 mg).
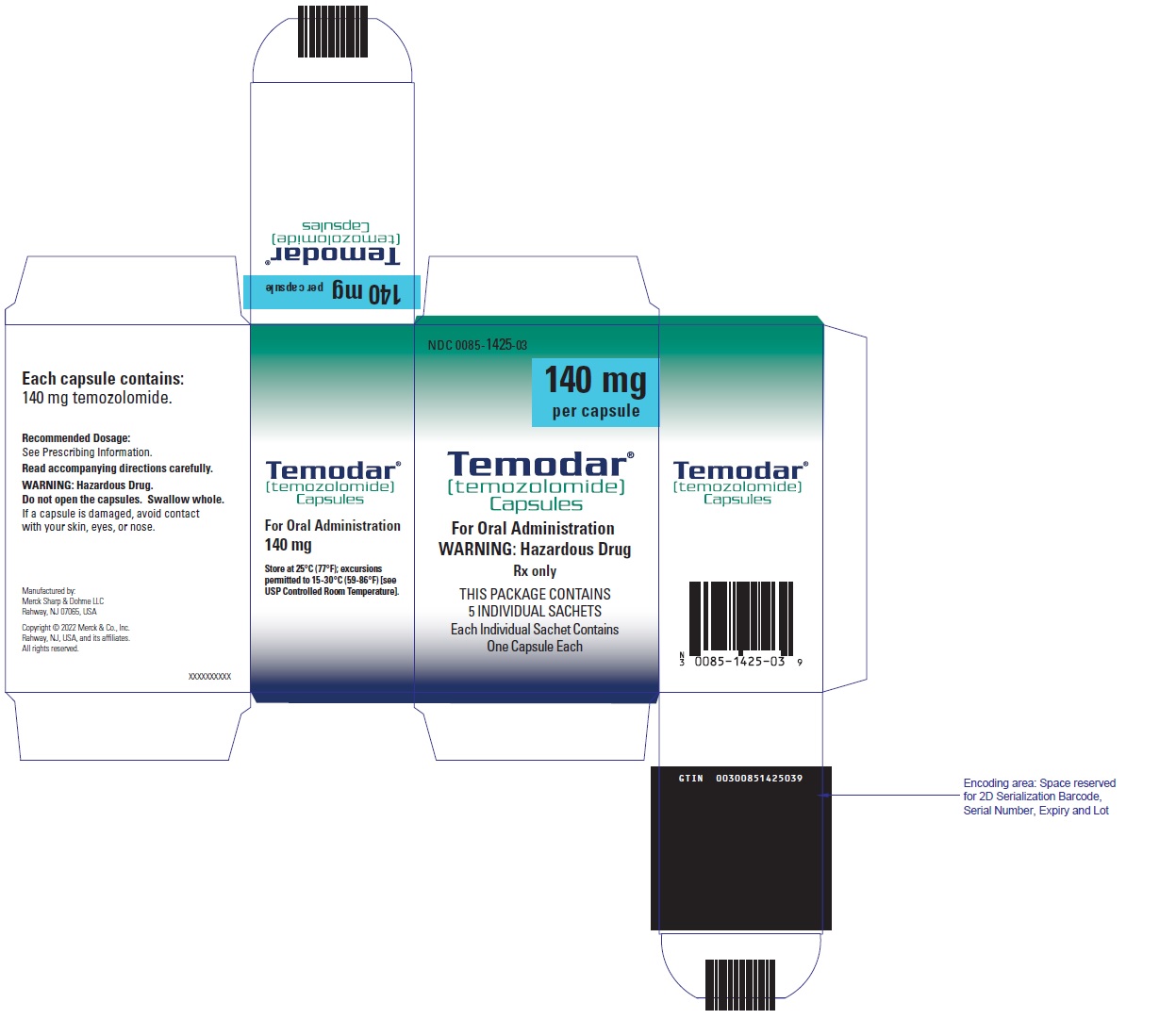
- TEMODAR 140 mg: lactose anhydrous (246 mg), colloidal silicon dioxide (0.4 mg), sodium starch glycolate (21 mg), tartaric acid (4.2 mg), and stearic acid (8.4 mg).
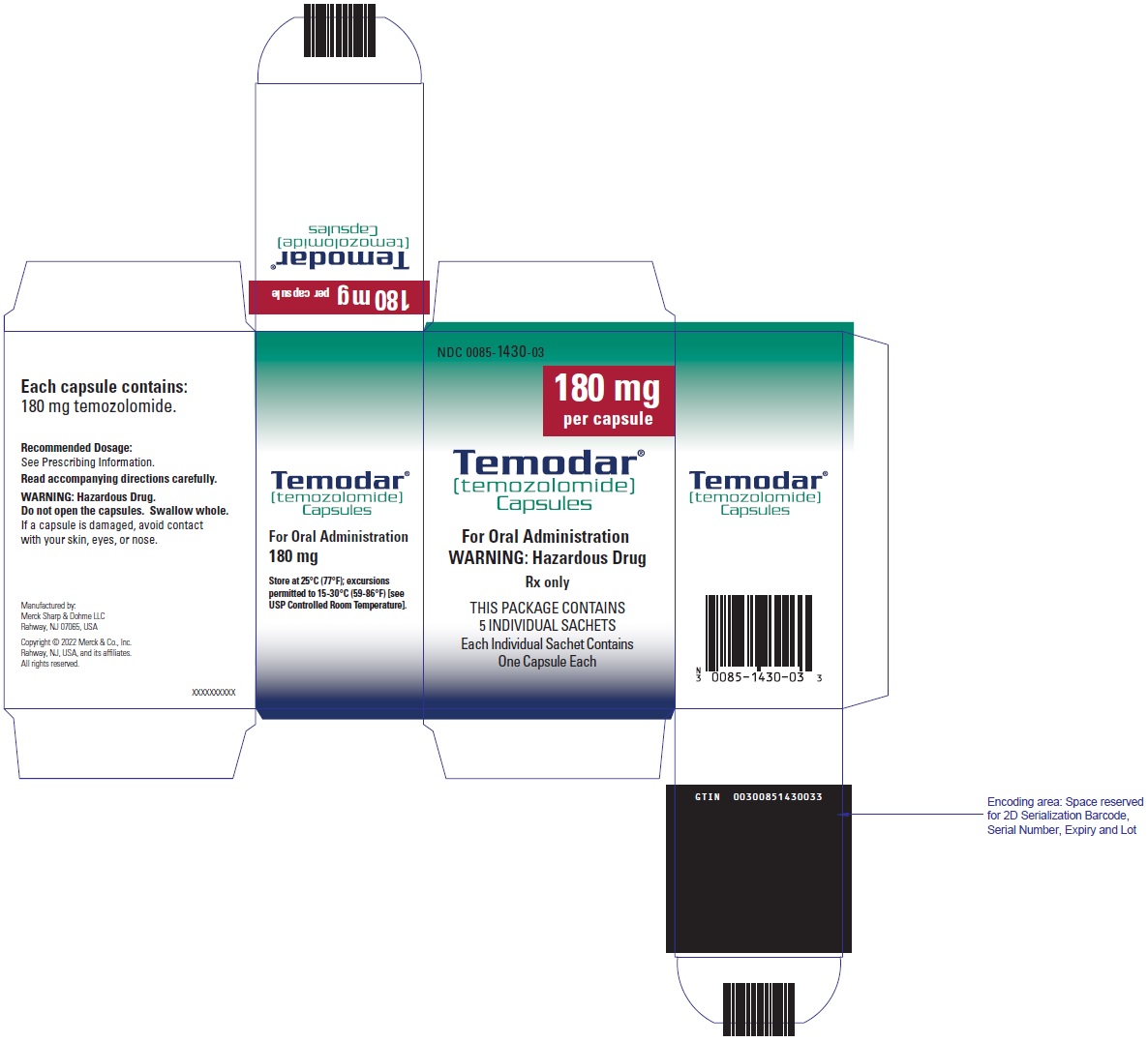
- TEMODAR 180 mg: lactose anhydrous (316.3 mg), colloidal silicon dioxide (0.5 mg), sodium starch glycolate (27 mg), tartaric acid (5.4 mg), and stearic acid (10.8 mg).
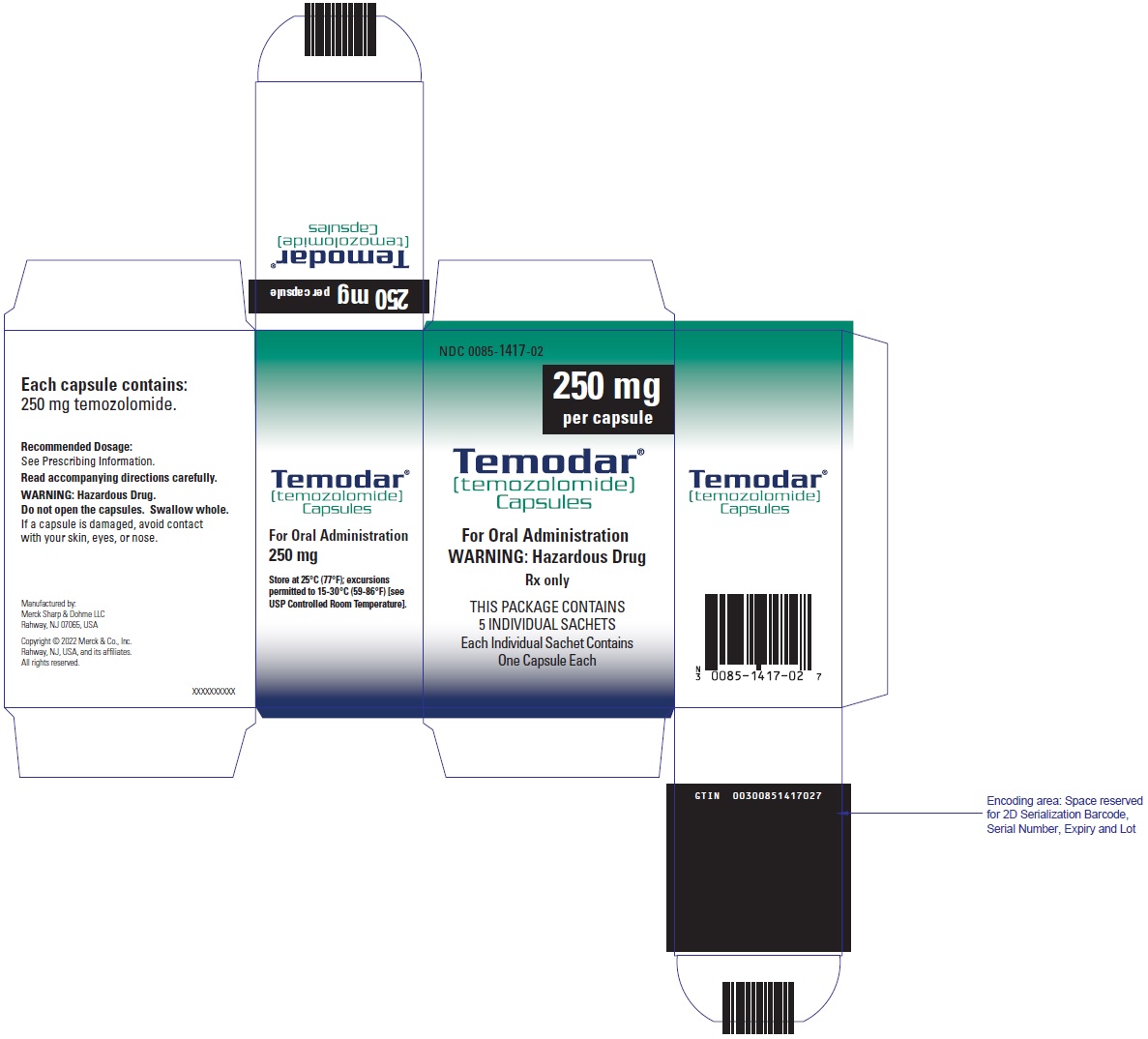
- TEMODAR 250 mg: lactose anhydrous (154.3 mg), colloidal silicon dioxide (0.7 mg), sodium starch glycolate (22.5 mg), tartaric acid (9 mg), and stearic acid (13.5 mg).
- TEMODAR 5 mg: The green cap contains gelatin, titanium dioxide, iron oxide yellow, sodium lauryl sulfate, and FD&C Blue #2.
- TEMODAR 20 mg: The yellow cap contains gelatin, sodium lauryl sulfate, and iron oxide yellow.
- TEMODAR 100 mg: The pink cap contains gelatin, titanium dioxide, sodium lauryl sulfate, and iron oxide red.
- TEMODAR 140 mg: The blue cap contains gelatin, sodium lauryl sulfate, and FD&C Blue #2.
- TEMODAR 180 mg: The orange cap contains gelatin, iron oxide red, iron oxide yellow, titanium dioxide, and sodium lauryl sulfate.
- TEMODAR 250 mg: The white cap contains gelatin, titanium dioxide, and sodium lauryl sulfate.
- “OSHA Hazardous Drugs.” OSHA.
[temozolomide]
Capsules
[temozolomide]
Capsules
[temozolomide]
Capsules
[temozolomide]
Capsules
[temozolomide]
Capsules
[temozolomide]
Capsules
[temozolomide]
for Injection