Brand Name
Savella
Generic Name
Milnacipran
View Brand Information FDA approval date: April 17, 2009
Classification: Serotonin and Norepinephrine Reuptake Inhibitor
Form: Tablet, Kit
What is Savella (Milnacipran)?
Milnacipran HCl tablets are indicated for the management of fibromyalgia. Milnacipran HCl tablets are not approved for use in pediatric patients [see Use in Specific Populations.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Savella (milnacipran hydrochloride)
WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS
SAVELLA is a selective serotonin and norepinephrine reuptake inhibitor (SNRI), similar to some drugs used for the treatment of depression and other psychiatric disorders. Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of such drugs in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on SAVELLA should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. SAVELLA is not approved for use in the treatment of major depressive disorder. SAVELLA is not approved for use in pediatric patients [see Indications and Usage (1), Warnings and Precautions (5.1), Use in Specific Populations (8.4)].
1INDICATIONS AND USAGE
SAVELLA is indicated for the management of fibromyalgia.
SAVELLA is not approved for use in pediatric patients
2DOSAGE AND ADMINISTRATION
SAVELLA is given orally with or without food.
Taking SAVELLA with food may improve the tolerability of the drug.
2.1Recommended Dosing
The recommended dose of SAVELLA is 100 mg/day (50 mg twice daily).
Based on efficacy and tolerability dosing may be titrated according to the following schedule:
Day 1: 12.5 mg once
Days 2-3: 25 mg/day (12.5 mg twice daily)
Days 4-7: 50 mg/day (25 mg twice daily)
After Day 7: 100 mg/day (50 mg twice daily)
Based on individual patient response, the dose may be increased to 200 mg/day (100 mg twice daily).
Doses above 200 mg/day have not been studied.
Taper SAVELLA and do not abruptly discontinue after extended use
2.2Patients with Renal Insufficiency
No dosage adjustment is necessary in patients with mild renal impairment.
Use SAVELLA with caution in patients with moderate renal impairment.
For patients with severe renal impairment (indicated by an estimated creatinine clearance of 5-29 mL/min), reduce the maintenance dose by 50% to 50 mg/day (25 mg twice daily).
Based on individual patient response, the dose may be increased to 100 mg/day (50 mg twice daily).
SAVELLA is not recommended for patients with end-stage renal disease.
2.3Patients with Hepatic Insufficiency
No dosage adjustment is necessary for patients with hepatic impairment.
As with any drug, exercise caution in patients with severe hepatic impairment.
2.4Discontinuing SAVELLA
Withdrawal symptoms have been observed in clinical trials following discontinuation of milnacipran, as with other serotonin and norepinephrine re-uptake inhibitors (SNRIs) and selective serotonin re-uptake inhibitors (SSRIs). Monitor patients for these symptoms when discontinuing treatment. Taper SAVELLA and do not abruptly discontinue after extended use
2.5Switching a Patient to or from a Monoamine Oxidase Inhibitor (MAOI)Intended to Treat Psychiatric Disorders
At least 14 days should elapse between discontinuation of a MAOI intended to treat psychiatric disorders and initiation of therapy with SAVELLA. Conversely, allow at least 5 days after stopping SAVELLA before starting a MAOI intended to treat psychiatric disorders
2.6Use of SAVELLAwith other MAOIs such as Linezolid or Methylene Blue
Do not start SAVELLA in a patient being treated with linezolid or intravenous methylene blue because there is increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, consider other interventions, including hospitalization
In some cases, a patient already receiving SAVELLA therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, discontinue SAVELLA promptly, and consider administering linezolid or intravenous methylene blue. Monitor the patient for symptoms of serotonin syndrome for 5 days or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with SAVELLA may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with SAVELLA is unclear. The clinician should nevertheless be aware of the possibility of emergent symptoms of serotonin syndrome with such use
3DOSAGE FORMS AND STRENGTHS
Film-coated, immediate-release tablets in four strengths: 12.5 mg, 25 mg, 50 mg, and 100 mg of milnacipran hydrochloride.
12.5 mg tablets are round, blue, "F" on one side, "L" on the reverse side;
25 mg tablets are round, white, "FL" on one side, "25" on the reverse side;
50 mg tablets are oval, white, "FL" on one side, "50" on the reverse side;
100 mg tablets are oval, pink, "FL" on one side, "100" on the reverse side.
4DRUG INTERACTIONS
Milnacipran undergoes minimal CYP450 related metabolism, with the majority of the dose excreted unchanged in urine (55%) and has a low binding to plasma proteins (13%). In vitro and in vivo studies showed that SAVELLA is unlikely to be involved in clinically significant pharmacokinetic drug interactions
4.1Monoamine Oxidase Inhibitors(MAOIs)
The concomitant use of SSRIs and SNRIs, including SAVELLA, with MAOIs increases the risk of serotonin syndrome. The use of MAOIs intended to treat psychiatric disorders with SAVELLA or within 5 days of stopping treatment with SAVELLA is contraindicated. The use of SAVELLA within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated. In addition, do not initiate SAVELLA in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with a MAOI such as linezolid or intravenous methylene blue in a patient taking SAVELLA, discontinue SAVELLA before initiating treatment with the MAOI
4.2Serotonergic Drugs
Serotonin syndrome can occur with use of SAVELLA and other serotonergic drugs (other SNRIs, SSRIs, triptans, tricyclic antidepressants, opioids, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort), or with drugs that impair metabolism of serotonin (i.e., monoamine oxidase inhibitors). Advise patients of the signs and symptoms associated with serotonin syndrome and to seek medical care immediately if they experience these symptoms
4.3Triptans
There have been rare postmarketing reports of serotonin syndrome with use of an SSRI and a triptan. If concomitant treatment of SAVELLA with a triptan is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases
4.4Catecholamines
SAVELLA inhibits the reuptake of norepinephrine. Therefore, concomitant use of SAVELLA with epinephrine and norepinephrine may be associated with paroxysmal hypertension and possible arrhythmia
4.5CNS-active drugs
Given the primary CNS effects of SAVELLA, use caution when it is taken in combination with other centrally acting drugs, including those with a similar mechanism of action.
Clomipramine: In a drug-drug interaction study, an increase in euphoria and postural hypotension was observed in patients who switched from clomipramine to SAVELLA.
4.6Clinically Important Interactions with Select Cardiovascular Agents
Digoxin: Use of SAVELLA concomitantly with digoxin may be associated with potentiation of adverse hemodynamic effects. Postural hypotension and tachycardia have been reported in combination therapy with intravenously administered digoxin (1 mg). Avoid co-administration of SAVELLA and intravenous digoxin
Clonidine: Because SAVELLA inhibits norepinephrine reuptake, co-administration with clonidine may inhibit clonidine’s anti-hypertensive effect.
4.7Drugs that Interfere with Hemostasis
Concomitant use of SAVELLA with an antiplatelet or anticoagulant drug (e.g., NSAIDs, aspirin, and warfarin) may potentiate the risk of bleeding. This may be due to the effect of SAVELLA on the release of serotonin by platelets. Closely monitor for bleeding for patients receiving an antiplatelet or anticoagulant drug when SAVELLA is initiated or discontinued
5OVERDOSAGE
Clinical Presentation
There is limited clinical experience with SAVELLA overdose in humans. In clinical trials, cases of acute ingestions up to 1000 mg, alone or in combination with other drugs, were reported with none being fatal.
In postmarketing experience, fatal outcomes have been reported for acute overdoses primarily involving multiple drugs but also with SAVELLA only. The most common signs and symptoms included increased blood pressure, cardio-respiratory arrest, changes in the level of consciousness (ranging from somnolence to coma), confusional state, dizziness, and increased hepatic enzymes.
Management of Overdose
There is no specific antidote to SAVELLA, but if serotonin syndrome ensues, specific treatment (such as with cyproheptadine and/or temperature control) may be considered. In case of acute overdose, treatment should consist of those general measures employed in the management of overdose with any drug.
Ensure adequate airway, oxygenation, and ventilation and monitor cardiac rhythm and vital signs. Induction of emesis is not recommended. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion or in symptomatic patients. Because there is no specific antidote for SAVELLA, consider symptomatic care and treatment with gastric lavage and activated charcoal as soon as possible for patients who experience a SAVELLA overdose.
Due to the large volume of distribution of this drug, forced diuresis, dialysis, hemoperfusion, and exchange transfusion are unlikely to be beneficial.
In managing overdose, consider the possibility of multiple drug involvement. The physician should consider contacting a poison control center for additional information on the treatment of any overdose. Telephone numbers for certified poison control centers are listed in the Physicians’ Desk Reference (PDR).
6DESCRIPTION
Milnacipran hydrochloride is a selective norepinephrine and serotonin reuptake inhibitor; it inhibits norepinephrine uptake with greater potency than serotonin. It is a racemic mixture with the chemical name: (±)-[1R(S),2S(R)]-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide hydrochloride. The structural formula is:
![The structural formula is Milnacipran hydrochloride is a selective norepinephrine and serotonin reuptake inhibitor; it inhibits norepinephrine uptake with greater potency than serotonin. It is a racemic mixture with the chemical name: (±)-[1R(S),2S(R)]-2-(aminomethyl)-N,N-diethyl-1-phenylcyclopropanecarboxamide hydrochloride.](https://dailymed.nlm.nih.gov/dailymed/image.cfm?name=savella-01.jpg&setid=16a4a314-f97e-4e91-95e9-576a3773d284)
Milnacipran hydrochloride is a white to off-white crystalline powder with a melting point of 179°C.
It is freely soluble in water, methanol, ethanol, chloroform, and methylene chloride and sparingly soluble in diethyl ether. It has an empirical formula of C15H23ClN2O and a molecular weight of 282.8 g/mol.
SAVELLA is available for oral administration as film-coated tablets containing 12.5 mg, 25 mg, 50 mg, and 100 mg milnacipran hydrochloride. Each tablet also contains dibasic calcium phosphate, povidone, carboxymethylcellulose calcium, colloidal silicon dioxide, magnesium stearate, and talc as inactive ingredients. The film coat contains the following additional inactive ingredients:
12.5 mg:
FD&C Blue #2 Aluminum Lake, polyvinyl alcohol, polyethylene glycol, titanium dioxide
25 mg:
Polyvinyl alcohol, polyethylene glycol, titanium dioxide
50 mg:
Polyvinyl alcohol, polyethylene glycol, titanium dioxide
100 mg:
FD&C Red #40 Aluminum Lake, polyvinyl alcohol, polyethylene glycol, titanium dioxide
7CLINICAL STUDIES
Management of Fibromyalgia
The efficacy of SAVELLA for the management of fibromyalgia was established in two double-blind, placebo-controlled, multicenter studies in adult patients (18-74 years of age). Enrolled patients met the American College of Rheumatology (ACR) criteria for fibromyalgia (a history of widespread pain for 3 months and pain present at 11 or more of the 18 specific tender point sites). Approximately 35% of patients had a history of depression. Study 1 was six months in duration and Study 2 was three months in duration.
A larger proportion of patients treated with SAVELLA than with placebo experienced a simultaneous reduction in pain from baseline of at least 30% (VAS) and also rated themselves as much improved or very much improved based on the patient global assessment (PGIC). In addition, a larger proportion of patients treated with SAVELLA met the criteria for treatment response, as measured by the composite endpoint that concurrently evaluated improvement in pain (VAS), physical function (SF-36 PCS), and patient global assessment (PGIC), in fibromyalgia as compared to placebo.
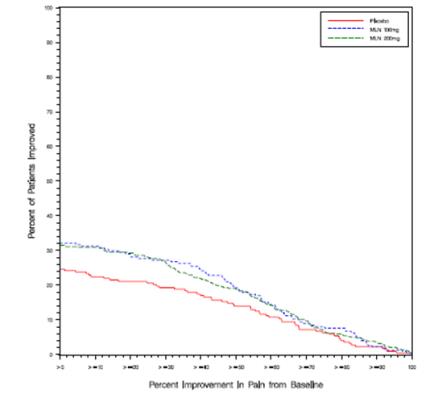
Study 1: This 6-month study compared total daily doses of SAVELLA 100 mg and 200 mg to placebo. Patients were enrolled with a minimum mean baseline pain score of ≥ 50 mm on a 100 mm visual analog scale (VAS) ranging from 0 (“no pain”) to 100 (“worst possible pain”). The mean baseline pain score in this trial was 69. The efficacy results for Study 1 are summarized in
Figure 1 shows the proportion of patients achieving various degrees of improvement in pain from baseline to the 3-month time point and who concurrently rated themselves globally improved (PGIC score of 1 or 2). Patients who did not complete the 3-month assessment were assigned 0% improvement. More patients in the SAVELLA treatment arms experienced at least a 30% reduction in pain from baseline (VAS) and considered themselves globally improved (PGIC) than did patients in the placebo arm. Treatment with SAVELLA 200 mg/day did not confer greater benefit than treatment with SAVELLA 100 mg/day.
Figure 1:Patients Achieving Various Levels of Pain Relief with Concurrent Ratings of Being Much or Very Much Improved on the PGIC ― Study 1
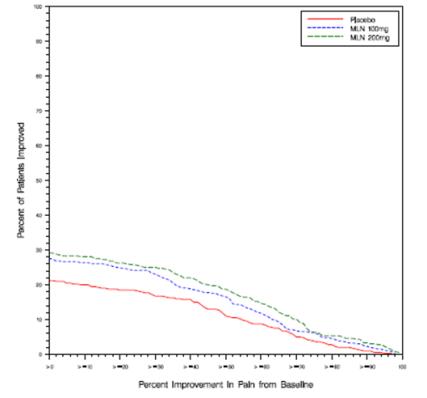
Study 2: This 3-month study compared total daily doses of SAVELLA 100 mg and 200 mg to placebo. Patients were enrolled with a minimum mean baseline pain score of ≥ 40 mm on a 100-mm VAS ranging from 0 (“no pain”) to 100 (“worst possible pain”). The mean baseline pain score in this trial was 65. The efficacy results for Study 2 are summarized in
Figure 2 shows the proportion of patients achieving various degrees of improvement in pain from baseline to the 3-month time point and who concurrently rated themselves globally improved (PGIC score of 1 or 2). Patients who did not complete the 3-month assessment were assigned 0% improvement. More patients in the SAVELLA treatment arms experienced at least a 30% reduction in pain from baseline (VAS) and considered themselves globally improved (PGIC) than did patients in the placebo arm. Treatment with SAVELLA 200 mg/day did not confer greater benefit than treatment with SAVELLA 100 mg/day.
Figure 2:Patients Achieving Various Levels of Pain Relief with Concurrent Ratings of BeingMuch or Very Much Improved on the PGIC ― Study 2
In both studies, some patients who rated themselves as globally “much” or “very much” improved experienced a decrease in pain as early as week 1 of treatment with a stable dose of SAVELLA that persisted throughout these studies.
8HOW SUPPLIED/STORAGE AND HANDLING
12.5-mg tablets:
Blue, round, film-coated tablets, debossed with “F” on one side and “L” on the reverse side
Bottles of 60: NDC 0456-1512-60
10X10 Unit Dose: NDC 0456-1512-63
25-mg tablets:
White, round, film-coated tablets, debossed with “FL” on one side and “25” on the reverse side
Bottles of 60: NDC 0456-1525-60
10X10 Unit Dose: NDC 0456-1525-63
50-mg tablets:
White, oval-shaped, film-coated tablets, debossed with “FL” on one side and “50” on the reverse side
Bottles of 60: NDC 0456-1550-60
10X10 Unit Dose: NDC 0456-1550-63
100-mg tablets:
Pink, oval-shaped, film-coated tablets, debossed with “FL” on one side and “100” on the reverse side
Bottles of 60: NDC 0456-1510-60
10X10 Unit Dose: NDC 0456-1510-63
Titration Pack:
4-Week Titration Pack: NDC 0456-1500-55
Blister package containing 55 tablets: 5 x 12.5-mg tablets, 8 x 25-mg tablets, and 42 x 50 mg tablets.
Storage
Store at 25°C (77°F); excursions permitted between 15°C and 30°C (between 59°F and 86°F)
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Advise patients of the following issues and to alert their prescriber if these occur while taking SAVELLA:
Clinical Worsening and Suicide Risk
Advise patients, their families, and their caregivers that patients with depression may be at increased risk for clinical worsening and/or suicidal ideation if they stop taking anti-depressant medication, change the dose, or start a new medication.
Encourage patients, their families, and their caregivers to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, or other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during treatment with SAVELLA or other drugs that inhibit the reuptake of norepinephrine and/or serotonin, and when the dose is adjusted up or down. Advise families and caregivers to observe for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt and to report such symptoms to the patient’s prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient’s presenting symptoms
Serotonin Syndrome
Inform patients about the risk of serotonin syndrome with use of SAVELLA as well as the increased risk when taken concomitantly with other serotonergic drugs including triptans, tricyclic antidepressants, opioids, lithium, tryptophan, buspirone, amphetamines and St. John’s Wort, and with drugs that impair metabolism of serotonin (in particular MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid)
Advise patients of the signs and symptoms associated with serotonin syndrome and to seek medical care immediately if they experience these symptoms.
Elevated Blood Pressure and Heart Rate
Advise patients that SAVELLA may increase their blood pressure and heart rate and that they should have their blood pressure and heart rate monitored at regular intervals when receiving treatment with SAVELLA
Increased Risk of Bleeding
Advise patients that the concomitant use of drugs that interfere with serotonin reuptake, including SAVELLA, and NSAIDs, aspirin, or anticoagulants has been associated with an increased risk of abnormal bleeding
Angle Closure Glaucoma
Advise patients that taking SAVELLA can cause mild pupillary dilation which, in susceptible individuals, can lead to an episode of angle closure glaucoma.
Ability to Drive and Use Machinery
Advise patients not to operate machinery or drive motor vehicles until they are reasonably certain that SAVELLA treatment does not affect their ability to engage in such activities.
Alcohol
Inform patients of the risks associated with drinking alcohol while taking SAVELLA
Sexual Dysfunction
Advise patients that use of SAVELLA may cause symptoms of sexual dysfunction in both male and female patients. Inform patients that they should discuss any changes in sexual function and potential management strategies with their healthcare provider
Discontinuation
Advise patients that withdrawal symptoms can occur when discontinuing treatment with SAVELLA, particularly when discontinuation is abrupt
Missing a Dose
Advise patients that if they miss a dose, they should skip the missed dose and take the next dose at their regular time.
Pregnancy
- Advise pregnant women to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with SAVELLA.
- Advise patients that SAVELLA may increase the risk of neonatal complications requiring prolonged hospitalization, respiratory support, and tube feeding.
Lactation
Advise breastfeeding women using SAVELLA to monitor infants for sedation, agitation, irritability, poor feeding, and poor weight gain and to seek medical care if they notice these signs
Distributed by:
AbbVie Inc.
1 N Waukegan Rd.
North Chicago, IL 60064
Licensed from Pierre Fabre Medicament
© 2025 AbbVie. All rights reserved.
SAVELLA and its design are trademarks of Allergan Sales, LLC, an AbbVie company.
20091024
10PRINCIPAL DISPLAY PANEL
Rx Only
11PRINCIPAL DISPLAY PANEL
Rx Only
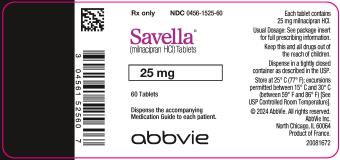
12PRINCIPAL DISPLAY PANEL
Rx Only
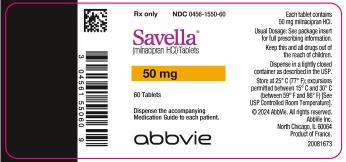
13PRINCIPAL DISPLAY PANEL
Rx Only
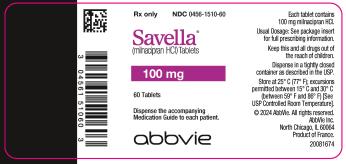
14PRINCIPAL DISPLAY PANEL
NDC 0456-1500-55
- Five 12.5 mg tablets
- Eight 25 mg tablets
- Forty-two 50 mg tablets
Dispense the enclosed
Medication Guide to each patient.
For additional information, visit www.savella.com
Rx only
abbvie
Medication Guide to each patient.
For additional information, visit www.savella.com
Rx only
abbvie