Brand Name
Pomalyst
Generic Name
Pomalidomide
View Brand Information FDA approval date: February 18, 2013
Classification: Thalidomide Analog
Form: Capsule
What is Pomalyst (Pomalidomide)?
POMALYST is a thalidomide analogue indicated for the treatment of adult patients: in combination with dexamethasone, for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor and have demonstrated disease progression on or within 60 days of completion of the last therapy.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Pomalyst (pomalidomide)
1DOSAGE FORMS AND STRENGTHS
- Capsules:1 mg, dark blue opaque cap and yellow opaque body, imprinted "POML" on the cap in white ink and "1 mg" on the body in black ink
- 2 mg, dark blue opaque cap and orange opaque body, imprinted "POML" on the cap and "2 mg" on the body in white ink
- 3 mg, dark blue opaque cap and green opaque body, imprinted "POML" on the cap and "3 mg" on the body in white ink
- 4 mg, dark blue opaque cap and blue opaque body, imprinted "POML" on the cap and "4 mg" on the body in white ink
2ADVERSE REACTIONS
The following clinically significant adverse reactions are described in detail in other labeling sections:
- Embryo-Fetal Toxicity
- Venous and Arterial Thromboembolism
- Increased Mortality in Patients with Multiple Myeloma When Pembrolizumab Is Added to a Thalidomide Analogue and Dexamethasone
- Hematologic Toxicity
- Hepatotoxicity
- Severe Cutaneous Reactions
- Dizziness and Confusional State
- Neuropathy
- Risk of Second Primary Malignancies
- Tumor Lysis Syndrome
- Hypersensitivity
2.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
2.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of POMALYST. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Pancytopenia
Endocrine Disorders: Hypothyroidism, hyperthyroidism
Gastrointestinal Disorders: Gastrointestinal hemorrhage
Hepatobiliary Disorders: Hepatic failure (including fatal cases), elevated liver enzymes
Immune system Disorders: Allergic reactions (e.g., angioedema, anaphylaxis, urticaria), solid organ transplant rejection
Infections and Infestations: Hepatitis B virus reactivation, Herpes zoster, progressive multifocal leukoencephalopathy (PML)
Neoplasms benign, malignant and unspecified (incl cysts and polyps): Tumor lysis syndrome, basal cell carcinoma, and squamous cell carcinoma of the skin
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson Syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS)
3OVERDOSAGE
Hemodialysis can remove pomalidomide from circulation.
4DESCRIPTION
Pomalidomide is a thalidomide analog. The chemical name is (RS)-4-Amino-2-(2,6-dioxo-piperidin-3-yl)-isoindoline-1,3-dione and it has the following chemical structure:
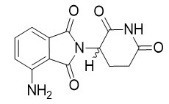
The empirical formula for pomalidomide is C
Pomalidomide is a yellow solid powder. It has limited to low solubility into organic solvents and it has low solubility in all pH solutions (about 0.01 mg/mL). Pomalidomide has a chiral carbon atom which exists as a racemic mixture of the R(+) and S(-) enantiomers.
POMALYST is available in 1-mg, 2-mg, 3-mg, and 4-mg capsules for oral administration. Each capsule contains pomalidomide as the active ingredient and the following inactive ingredients: mannitol, pregelatinized starch, and sodium stearyl fumarate. The 1-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, white ink, and black ink. The 2-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, FD&C red 3, and white ink. The 3-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 2, yellow iron oxide, and white ink. The 4-mg capsule shell contains gelatin, titanium dioxide, FD&C blue 1, FD&C blue 2, and white ink.
5REFERENCES
- OSHA Hazardous Drugs.
6HOW SUPPLIED/STORAGE AND HANDLING
Dark blue opaque cap and yellow opaque body, imprinted "POML" on the cap in white ink and "1 mg" on the body in black ink
Dark blue opaque cap and orange opaque body, imprinted "POML" on the cap and "2 mg" on the body in white ink
Dark blue opaque cap and green opaque body, imprinted "POML" on the cap and "3 mg" on the body in white ink
Dark blue opaque cap and blue opaque body, imprinted "POML" on the cap and "4 mg" on the body in white ink
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
8POMALYST 1 mg Representative Packaging
NDC 59572-501-21

9POMALYST 2 mg Representative Packaging
NDC 59572-502-21
10POMALYST 3 mg Representative Packaging
NDC 59572-503-21

11POMALYST 4 mg Representative Packaging
NDC 59572-504-21
