Generic Name
Semaglutide
Brand Names
Ozempic, Wegovy, Rybelsus
FDA approval date: September 20, 2019
Classification: GLP-1 Receptor Agonist
Form: Injection, Tablet
What is Ozempic (Semaglutide)?
OZEMPIC is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus., to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Ozempic (semaglutide)
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether OZEMPIC causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- OZEMPIC is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
1INDICATIONS AND USAGE
OZEMPIC is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) in adults with type 2 diabetes mellitus and established cardiovascular disease.
Limitations of Use
- OZEMPIC has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis
- OZEMPIC is not indicated for use in patients with type 1 diabetes mellitus.
2DOSAGE FORMS AND STRENGTHS
Injection: clear, colorless solution available in 3 pre-filled, disposable, single-patient-use pens:
The 2 mg/1.5 mL (1.34 mg/mL) strength is not currently marketed by Novo Nordisk Inc.
3CONTRAINDICATIONS
OZEMPIC is contraindicated in patients with:
- A personal or family history of MTC or in patients with MEN 2
- A serious hypersensitivity reaction to semaglutide or to any of the excipients in OZEMPIC. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with OZEMPIC
4ADVERSE REACTIONS
The following serious adverse reactions are described below or elsewhere in the prescribing information:
• Risk of Thyroid C-cell Tumors
• Pancreatitis
• Diabetic Retinopathy Complications
• Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
• Acute Kidney Injury
• Hypersensitivity
• Acute Gallbladder Disease
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Trials
The data in Table 1 are derived from 2 placebo-controlled trials (1 monotherapy trial and 1 trial in combination with basal insulin) in patients with type 2 diabetes
Pool of Placebo- and Active-Controlled Trials
The occurrence of adverse reactions was also evaluated in a larger pool of patients with type 2 diabetes
participating in 7 placebo- and active-controlled glycemic control trials
Common Adverse Reactions
Table 1 shows common adverse reactions, excluding hypoglycemia, associated with the use of OZEMPIC in the pool of placebo-controlled trials. These adverse reactions occurred more commonly on OZEMPIC than on placebo and occurred in at least 5% of patients treated with OZEMPIC.
Table 1. Adverse Reactions in Placebo-Controlled Trials Reported in ≥5% of OZEMPIC-Treated Patients with Type 2 Diabetes Mellitus
In the pool of placebo- and active-controlled trials and in the 2-year cardiovascular outcomes trial, the types and frequency of common adverse reactions, excluding hypoglycemia, were similar to those listed in Table 1.
In a clinical trial with 959 patients treated with OZEMPIC 1 mg or OZEMPIC 2 mg once weekly as add-on to metformin with or without sulfonylurea treatment for 40 weeks, no new safety signals were identified.
Gastrointestinal Adverse Reactions
In the pool of placebo-controlled trials, gastrointestinal adverse reactions occurred more frequently among patients receiving OZEMPIC than placebo (placebo 15.3%, OZEMPIC 0.5 mg 32.7%, OZEMPIC 1 mg 36.4%). The majority of reports of nausea, vomiting, and/or diarrhea occurred during dose escalation. More patients receiving OZEMPIC 0.5 mg (3.1%) and OZEMPIC 1 mg (3.8%) discontinued treatment due to gastrointestinal adverse reactions than patients receiving placebo (0.4%).
In the trial with OZEMPIC 1 mg and 2 mg, gastrointestinal adverse reactions occurred more frequently among patients receiving OZEMPIC 2 mg (34.0%) vs OZEMPIC 1 mg (30.8%).
- In addition to the reactions in Table 1, the following gastrointestinal adverse reactions with a frequency of <5% were associated with OZEMPIC (frequencies listed, respectively, as: placebo; 0.5 mg; 1 mg): dyspepsia (1.9%, 3.5%, 2.7%), eructation (0%, 2.7%, 1.1%), flatulence (0.8%, 0.4%, 1.5%), gastroesophageal reflux disease (0%, 1.9%, 1.5%), and gastritis (0.8%, 0.8%, 0.4%).
Other Adverse Reactions
Hypoglycemia
Table 2 summarizes the incidence of events related to hypoglycemia by various definitions in the placebo-controlled trials.
Table 2. Hypoglycemia Adverse Reactions in Placebo-Controlled Trials in Patients with Type 2 Diabetes Mellitus
Hypoglycemia was more frequent when OZEMPIC was used in combination with a sulfonylurea
Injection Site Reactions
In placebo-controlled trials, injection site reactions (e.g., injection-site discomfort, erythema) were reported in 0.2% of OZEMPIC-treated patients.
Increases in Amylase and Lipase
In placebo-controlled trials, patients exposed to OZEMPIC had a mean increase from baseline in amylase of 13% and lipase of 22%. These changes were not observed in placebo-treated patients.
Cholelithiasis
In placebo-controlled trials, cholelithiasis was reported in 1.5% and 0.4% of patients-treated with OZEMPIC 0.5 mg and 1 mg, respectively. Cholelithiasis was not reported in placebo-treated patients.
Increases in Heart Rate
In placebo-controlled trials, OZEMPIC 0.5 mg and 1 mg resulted in a mean increase in heart rate of 2 to 3 beats per minute. There was a mean decrease in heart rate of 0.3 beats per minute in placebo-treated patients.
Fatigue, Dysgeusia and Dizziness
Other adverse reactions with a frequency of >0.4% were associated with OZEMPIC include fatigue, dysgeusia and dizziness.
4.2Immunogenicity
Consistent with the potentially immunogenic properties of protein and peptide pharmaceuticals, patients treated with OZEMPIC may develop anti-semaglutide antibodies. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, the incidence of antibodies to semaglutide in the studies described below cannot be directly compared with the incidence of antibodies in other studies or to other products.
Across the placebo- and active-controlled glycemic control trials, 32 (1.0%) OZEMPIC-treated patients developed anti-drug antibodies (ADAs) to the active ingredient in OZEMPIC (i.e., semaglutide). Of the 32 semaglutide-treated patients that developed semaglutide ADAs, 19 patients (0.6% of the overall population) developed antibodies cross-reacting with native GLP-1. The
4.3Postmarketing Experience
The following adverse reactions have been reported during post-approval use of semaglutide, the active ingredient of OZEMPIC. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: Ileus
Hypersensitivity: anaphylaxis, angioedema, rash, urticaria.
Hepatobiliary: cholecystitis, cholecystectomy
5OVERDOSAGE
In the event of overdose, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations. A prolonged period of observation and treatment for these symptoms may be necessary, taking into account the long half-life of OZEMPIC of approximately 1 week.
6DESCRIPTION
OZEMPIC (semaglutide) injection, for subcutaneous use, contains semaglutide, a human GLP-1 receptor agonist (or GLP-1 analog). The peptide backbone is produced by yeast fermentation. The main protraction mechanism of semaglutide is albumin binding, facilitated by modification of position 26 lysine with a hydrophilic spacer and a C18 fatty di-acid. Furthermore, semaglutide is modified in position 8 to provide stabilization against degradation by the enzyme dipeptidyl-peptidase 4 (DPP-4). A minor modification was made in position 34 to ensure the attachment of only one fatty di-acid. The molecular formula is C
Structural formula:
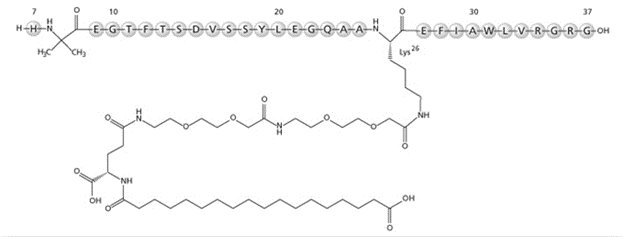
OZEMPIC is a sterile, aqueous, clear, colorless solution. Each 3 mL pre-filled single-patient use pen contains semaglutide 2 mg (0.68 mg/mL), 4 mg (1.34 mg/mL), or 8 mg (2.68 mg/mL). Each 1 mL of OZEMPIC solution also contains the following inactive ingredients: disodium phosphate dihydrate, 1.42 mg; propylene glycol, 14.0 mg; phenol, 5.50 mg; and water for injections. OZEMPIC has a pH of approximately 7.4. Hydrochloric acid or sodium hydroxide may be added to adjust pH. The 2 mg/1.5 mL (1.34 mg/mL) strength is not currently marketed by Novo Nordisk Inc.
7HOW SUPPLIED/STORAGE AND HANDLING
Product: 50090-5949
NDC: 50090-5949-0 3 mL in a SYRINGE, PLASTIC / 1 in a CARTON
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Risk of Thyroid C-cell Tumors
Inform patients that semaglutide causes thyroid C-cell tumors in rodents and that the human relevance of this finding has not been determined. Counsel patients to report symptoms of thyroid tumors (e.g., a lump in the neck, hoarseness, dysphagia, or dyspnea) to their physician
Pancreatitis
Inform patients of the potential risk for pancreatitis. Instruct patients to discontinue OZEMPIC promptly and contact their physician if pancreatitis is suspected (severe abdominal pain that may radiate to the back, and which may or may not be accompanied by vomiting)
Diabetic Retinopathy Complications
Inform patients to contact their physician if changes in vision are experienced during treatment with OZEMPIC
Never Share an OZEMPIC Pen Between Patients
Advise patients that they must never share an OZEMPIC pen with another person, even if the needle is changed, because doing so carries a risk for transmission of blood-borne pathogens
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
Inform patients that the risk of hypoglycemia is increased when OZEMPIC is used with an insulin secretagogue (such as a sulfonylurea) or insulin. Educate patients on the signs and symptoms of hypoglycemia
Acute Kidney Injury
Advise patients treated with OZEMPIC of the potential risk of dehydration due to gastrointestinal adverse reactions and take precautions to avoid fluid depletion. Inform patients of the potential risk for worsening renal function and explain the associated signs and symptoms of renal impairment, as well as the possibility of dialysis as a medical intervention if acute kidney injury occurs
Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions have been reported during postmarketing use of OZEMPIC. Advise patients on the symptoms of hypersensitivity reactions and instruct them to stop taking OZEMPIC and seek medical advice promptly if such symptoms occur
Acute Gallbladder Disease
Inform patients of the potential risk for cholelithiasis or cholecystitis. Instruct patients to contact their physician if cholelithiasis or cholecystitis is suspected for appropriate clinical follow-up
Pregnancy
Advise a pregnant woman of the potential risk to a fetus. Advise women to inform their healthcare provider if they are pregnant or intend to become pregnant
Missed doses
Inform patients if a dose is missed, it should be administered as soon as possible within 5 days after the missed dose. If more than 5 days have passed, the missed dose should be skipped and the next dose should be administered on the regularly scheduled day. In each case, inform patients to resume their regular once weekly dosing schedule
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd
Denmark
For information about OZEMPIC contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-888-693-6742
Version: 9
OZEMPIC® and NovoFine® are registered trademarks of Novo Nordisk A/S.
PATENT INFORMATION: http://www.novonordisk-us.com/products/product-patents.html
© 2023 Novo Nordisk
9Medication Guide
- This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 03/2022
10Instructions for Use – 2 mg dose, 3 mL pen
For more information go to
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd
Denmark
For information about OZEMPIC contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-888-693-6742
Version: 2
OZEMPIC
PATENT Information: https://novonordisk-us.com/products/product-patents.html
© 2023 Novo Nordisk
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: September 2023

11semaglutide
