Xeljanz
What is Xeljanz (Tofacitinib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is an observational prospective study with two years of follow-up, designed to evaluate the effectiveness of tofacitinib in patients with moderate to severe ulcerative colitis in French clinical practice
Summary: Xeljanz XR extended-release tablets 11 mg (Tofacitinib citrate) is a drug subject to the risk management plan in accordance with Article 4-1-11 of the Regulation on Safety of Medicinal Products, etc. in Korea. As part of additional pharmacovigilance activity, this Post-marketing Surveillance (PMS) was planned to evaluate safety and effectiveness of Xeljanz XR under routine clinical practice. At le...
Summary: This is an observational study where pregnant women treated with non-anti-TNF agents or targeted small molecules approved for IBD treatment will be included. Although it is a multicentre, nationwide study, the number of patients to be included is expected to be relatively low (in DUMBO 1, during 5 years of recruitment, 88 patients treated with ustekimunab, 34 treated with vedolizumab, and 2 expose...
Related Latest Advances
Brand Information
- Serious Infections
- Increased Risk of Mortality
- Malignancy and Lymphoproliferative Disorders
- Major Adverse Cardiovascular Events
- Thrombosis
- Gastrointestinal Perforations
- Hypersensitivity Reactions
- Laboratory Abnormalities
- 5 mg white round, immediate-release film-coated tablet. Each tablet contains 5 mg of tofacitinib (equivalent to 8.08 mg of tofacitinib citrate) and the following inactive ingredients: croscarmellose sodium, HPMC 2910/Hypromellose 6cP, lactose monohydrate, macrogol/PEG3350, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin.
- 10 mg blue round, immediate-release film-coated tablet. Each tablet contains 10 mg of tofacitinib (equivalent to 16.16 mg of tofacitinib citrate) and the following inactive ingredients: croscarmellose sodium, FD&C Blue #1/Brilliant Blue FCF Aluminum Lake, FD&C Blue #2/Indigo Carmine Aluminum Lake, HPMC 2910/Hypromellose 6cP, lactose monohydrate, macrogol/PEG3350, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin.
- 11 mg pink, oval, extended-release film-coated tablet with a drilled hole at one end of the tablet band. Each tablet contains 11 mg of tofacitinib (equivalent to 17.77 mg tofacitinib citrate) and the following inactive ingredients: cellulose acetate, copovidone, hydroxyethyl cellulose, hydroxypropyl cellulose, HPMC 2910/Hypromellose, magnesium stearate, red iron oxide, sorbitol, titanium dioxide and triacetin. Printing ink contains, ammonium hydroxide, ferrosoferric oxide/black iron oxide, propylene glycol, and shellac glaze.
- 22 mg beige, oval, extended-release film-coated tablet with a drilled hole at one end of the tablet band. Each tablet contains 22 mg of tofacitinib (equivalent to 35.54 mg tofacitinib citrate) and the following inactive ingredients: cellulose acetate, copovidone, FD&C Blue #2 Aluminum Lake, hydroxyethyl cellulose, hydroxypropyl cellulose, HPMC 2910/Hypromellose, magnesium stearate, red iron oxide, sorbitol, titanium dioxide, triacetin, and yellow iron oxide. Printing ink contains ammonium hydroxide, ferrosoferric oxide/black iron oxide, propylene glycol, and shellac glaze.
(tofacitinib)
oral solution
- Store XELJANZ oral solution at room temperature between 68°F to 77°F (20°C to 25°C).
- Always store XELJANZ oral solution in the original bottle and carton to protect from light.
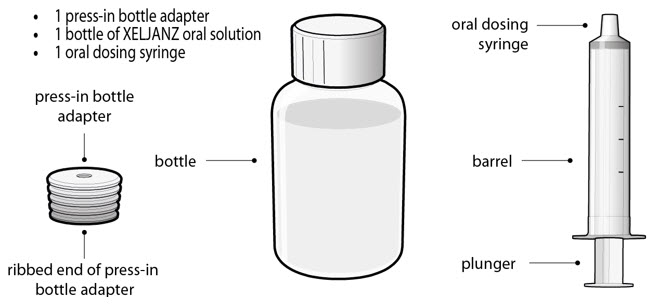
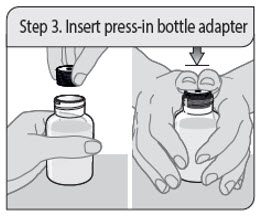
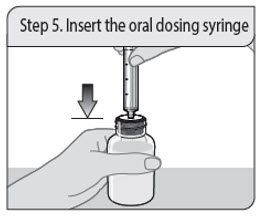
- 1 press-in bottle adapter
- 1 bottle of XELJANZ oral solution
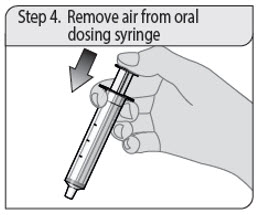
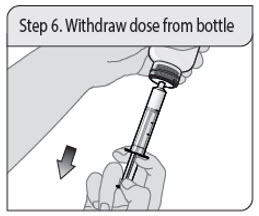
- 1 oral dosing syringe
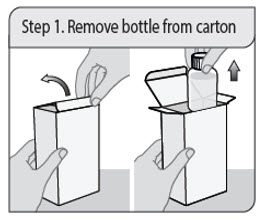
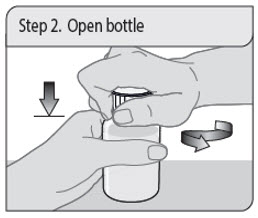
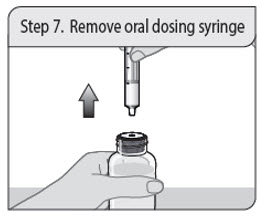
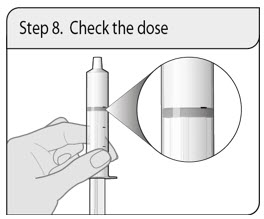
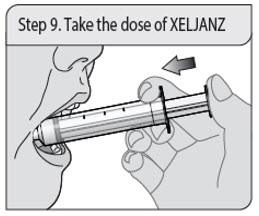
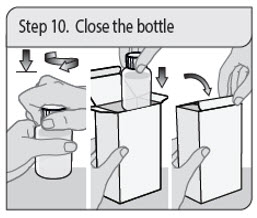
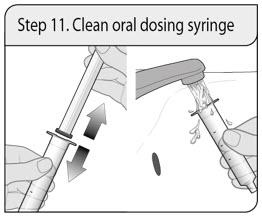

ALWAYS DISPENSE WITH MEDICATION GUIDE
(tofacitinib tablets)
ALWAYS DISPENSE WITH MEDICATION GUIDE
(tofacitinib) tablets

ALWAYS DISPENSE WITH MEDICATION GUIDE
(tofacitinib) tablets
Ulcerative Colitis



- Oral solution bottle
- 1 Oral dosing syringe
- 1 Press-in bottle adapter
- Prescribing Information
- Medication Guide
- Instructions for Use


