Mavyret
What is Mavyret (Glecaprevir)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: A double-blind randomized placebo-controlled trial to further investigate glecaprevir/pibrentasvir (GLE/PIB), a direct acting antiviral (DAA) that has been associated with posttraumatic stress disorder (PTSD) symptom improvement when prescribed for the treatment of chronic hepatitis C viral infection (HCV).
Summary: This is a prospective, non-blinded cohort study that will assess the safety, tolerability, and antiviral efficacy of glecaprevir/pibrentasivir therapy given post-discharge to HCV-negative recipients of HCV infected donors. Patients who meet entry criteria will be enrolled while on the transplant waitlist. At the time of transplant, some donors will be HCV positive / NAT positive and some will not ...
Summary: This study will evaluate the proportion of patients achieving confirmed SVR12 (undetectable HCV RNA at time point 12 weeks plus post treatment commencement) in patients hospitalised for IRID (injecting related infectious diseases) and commencing inpatient DAA treatment within public hospital services.
Related Latest Advances
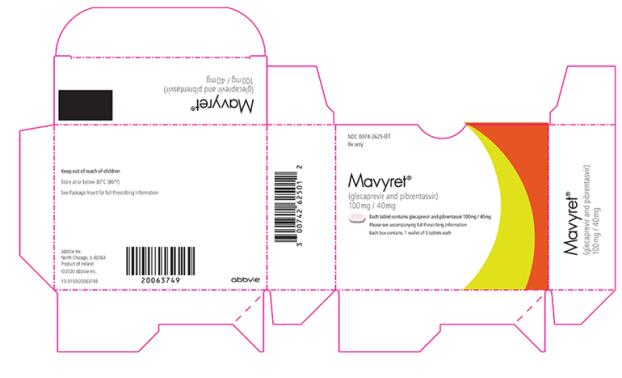
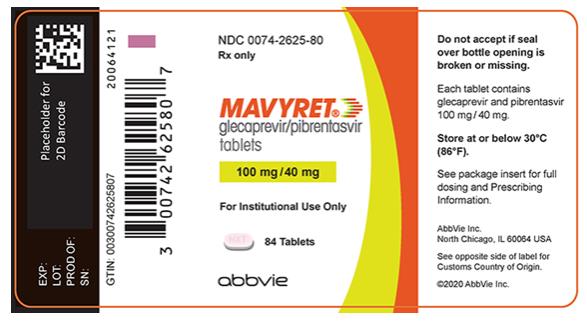
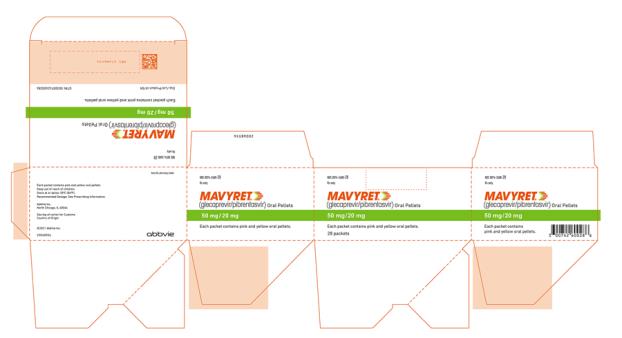
Brand Information
- Tablets: pink, oblong-shaped, film-coated, and debossed with “NXT” on one side. Each tablet contains 100 mg glecaprevir and 40 mg of pibrentasvir.
- Oral pellets: pink and yellow coated pellets in unit-dose packets. Each packet contains 50 mg glecaprevir and 20 mg pibrentasvir.
- MAVYRET is contraindicated in patients with moderate or severe hepatic impairment (Child-Pugh B or C) or those with any history of prior hepatic decompensation
- MAVYRET is contraindicated with atazanavir or rifampin
- Less than 18 hours from the usual time that MAVYRET should have been taken – advise the patient to take the dose as soon as possible and then to take the next dose at the usual time.
- More than 18 hours from the usual time that MAVYRET should have been taken – advise the patient not to take the missed dose and to take the next dose at the usual time.