Brand Name
Doxercalciferol
View Brand InformationFDA approval date: May 24, 2017
Classification: Vitamin D2 Analog
Form: Injection, Capsule
What is Doxercalciferol?
Doxercalciferol Injection is indicated for the treatment of secondary hyperparathyroidism in adult patients with CKD on dialysis. Doxercalciferol is a synthetic vitamin D 2 analog:, Doxercalciferol Injection is indicated for the treatment of secondary hyperparathyroidism in adult patients with CKD on dialysis.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Doxercalciferol (Doxercalciferol)
1INDICATIONS AND USAGE
- Doxercalciferol injection is indicated for the treatment of secondary hyperparathyroidism in adult patients with CKD on dialysis.
2DOSAGE FORMS AND STRENGTHS
Injection: Sterile, clear and colorless aqueous solution available as follows:
- 2 mcg/mL single-dose vial
- 4 mcg/2 mL (2 mcg/mL) single-dose vial
3CONTRAINDICATIONS
Doxercalciferol is contraindicated in patients with:
- Hypercalcemia
- Vitamin D toxicity
- Known hypersensitivity to doxercalciferol or any of the inactive ingredients of doxercalciferol injection; serious hypersensitivity reactions including anaphylaxis and angioedema have been reported
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in another section of the label:
- Hypercalcemia
- Serious Hypersensitivity Reactions
- Adynamic Bone Disease
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Doxercalciferol Capsules
Adverse reactions in patients with CKD on dialysis
Doxercalciferol capsules have been evaluated in two placebo-controlled, double-blind studies in patients with CKD on hemodialysis. Patients were treated with doxercalciferol capsules (n=61) or placebo (n=61). After randomization to two groups, eligible patients underwent an 8-week washout period during which no vitamin D derivatives were administered to either group. Subsequently, all patients received doxercalciferol capsules in an open-label fashion for 16 weeks followed by a double-blind period of 8 weeks during which patients received either doxercalciferol capsules or placebo. Adverse reactions occurring in the doxercalciferol capsule groups at a frequency of 2% or greater, and more frequently than in the placebo group are presented in Table 2.
Table 2: Adverse Reactions Occurring in ≥ 2% Doxercalciferol Capsule-Treated Patients with CKD on Dialysis and Greater than Placebo in Two Double-Blind Clinical Studies
Doxercalciferol Injection
Adverse reactions in patients with CKD on hemodialysis
Doxercalciferol injection has been studied in 70 patients with CKD on hemodialysis in two 12-week, open-label, single-arm, multicenter studies
There was no placebo group included in the studies of doxercalciferol injection. Adverse reactions in patients with CKD on hemodialysis receiving doxercalciferol injection are expected to be similar to those reported in placebo-controlled studies of doxercalciferol capsules presented in Table 2.
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of doxercalciferol injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or to establish a causal relationship to drug exposure.
Hypersensitivity reactions, including fatal outcome, have been reported in patients on hemodialysis following administration of doxercalciferol injection. Hypersensitivity reactions include anaphylaxis with symptoms of angioedema (involving face, lips, tongue and airways), hypotension, unresponsiveness, chest discomfort, shortness of breath, cardiopulmonary arrest, pruritus, and skin burning sensation.
5DRUG INTERACTIONS
Tables 3 include clinically significant drug interactions with doxercalciferol injection.
Table 3: Clinically Significant Drug Interactions with Doxercalciferol Injection
6OVERDOSAGE
Overdosage of doxercalciferol may lead to hypercalcemia, hypercalciuria, and hyperphosphatemia
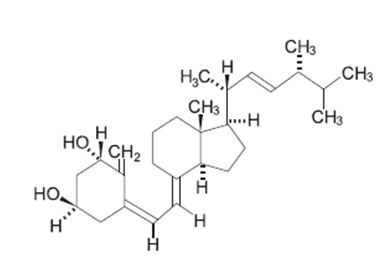
7DESCRIPTION
Doxercalciferol injection contains doxercalciferol, which is a synthetic vitamin D
Doxercalciferol, USP is a colorless crystalline compound with a calculated molecular weight of 412.66 and a molecular formula of C
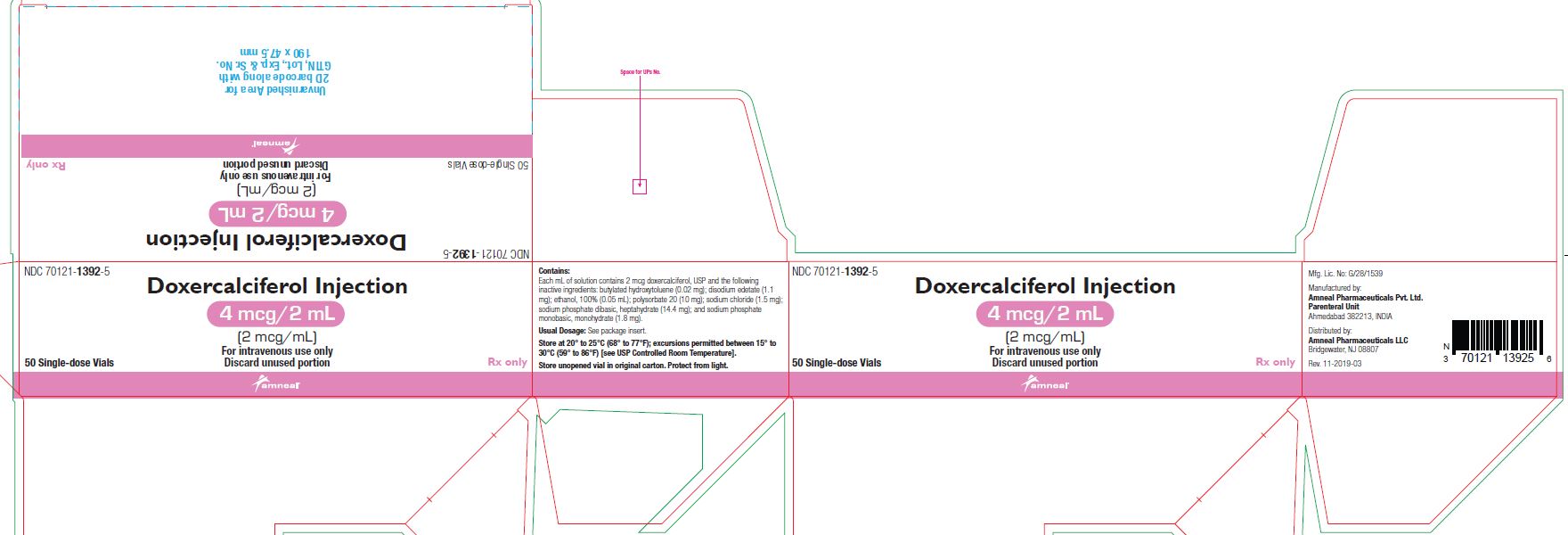
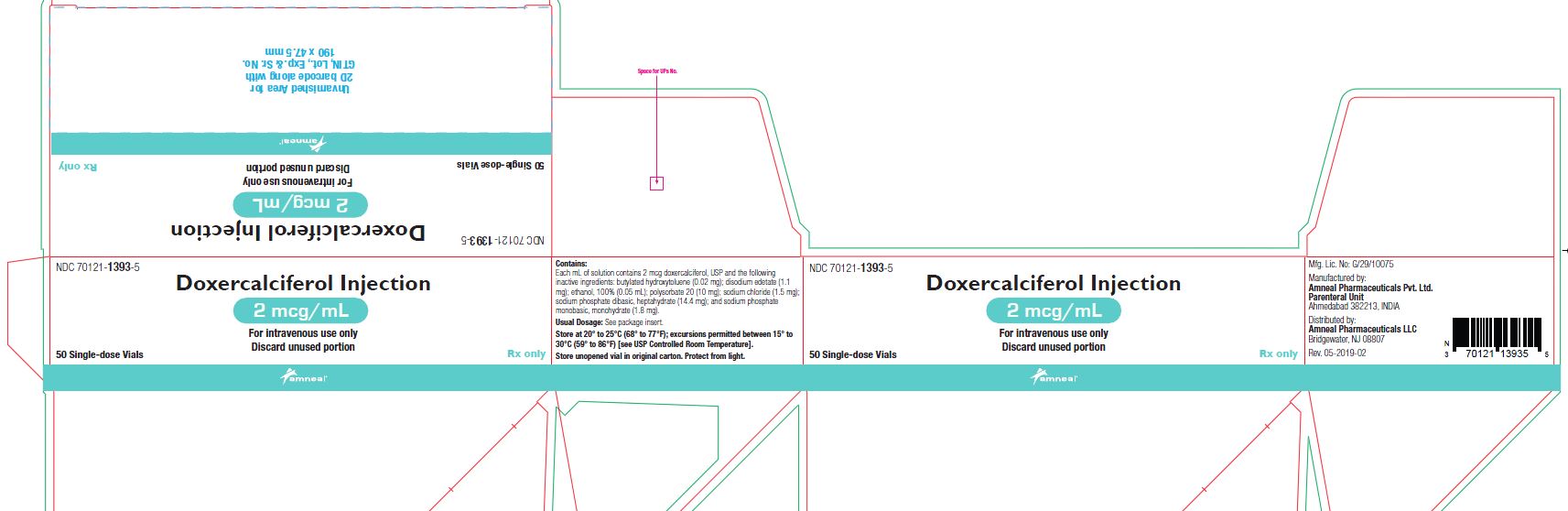
Doxercalciferol injection 1 mL single-dose vials contain 2 mcg/mL of doxercalciferol, USP. Doxercalciferol injection 2 mL single-dose vials contain 4 mcg/2 mL (2 mcg/mL) of doxercalciferol, USP. Each milliliter (mL) of solution contains 2 mcg doxercalciferol, USP and the following inactive ingredients: butylated hydroxytoluene (0.02 mg); disodium edetate (1.1 mg); ethanol, 100% (0.05 mL); polysorbate 20 (10 mg); sodium chloride (1.5 mg); sodium phosphate dibasic, heptahydrate (14.4 mg); and sodium phosphate monobasic, monohydrate (1.8 mg).
8HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Doxercalciferol Injection is a sterile, clear, colorless solution supplied in 2 mL amber glass vials containing
It is available as follows:
2 mcg/mL, 1 mL
1 mL, Single-dose Vial: NDC 70121-1393-1
2 mcg/mL, 2 mL
2 mL, Single-dose Vial: NDC 70121-1392-1
Storage and Handling
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Discard unused portion.
Protect from light. Store unopened vial in original carton.
9PATIENT COUNSELING INFORMATION
Hypercalcemia
Advise patients to contact a health care provider if they develop symptoms of elevated calcium (e.g., feeling tired, difficulty thinking clearly, loss of appetite, nausea, vomiting, constipation, increased thirst, increased urination and weight loss)
Hypersensitivity
Inform patients that hypersensitivity reactions can occur with doxercalciferol
Monitoring
Inform patients that they will need routine monitoring of laboratory parameters such as calcium and intact PTH while receiving doxercalciferol. Inform patients that more frequent monitoring is necessary during the initiation of therapy, following dose changes or when potentially interacting medications are started or discontinued
Drug Interactions
Advise patients to inform their physician of all medications, including prescription and nonprescription drugs, and supplements they are taking. Advise patients to also inform their physician that they are receiving doxercalciferol if a new medication is prescribed
Manufactured by:
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Bridgewater, NJ 08807
Rev. 12-2019-03
10PRINCIPAL DISPLAY PANEL
NDC 70121-1393-1
Doxercalciferol Injection, 2 mcg/mL
Rx Only
Vial Label
Amneal Pharmaceuticals LLC
NDC 70121-1393-6
Doxercalciferol Injection, 2 mcg/mL
Rx Only
25 single-dose Vial in a Carton
Amneal Pharmaceuticals LLC
NDC 70121-1393-5
Doxercalciferol Injection, 2 mcg/mL
Rx Only
50 single-dose Vial in a Carton
Amneal Pharmaceuticals LLC
NDC 70121-1392-1
Doxercalciferol Injection, 4 mcg/2 mL
Rx Only
Vial Label
Amneal Pharmaceuticals LLC
NDC 70121-1392-6
Doxercalciferol Injection, 4 mcg/2 mL
Rx Only
25 single-dose Vial in a Carton
AmnealPharmaceuticals LLC
NDC 70121-1392-5
Doxercalciferol Injection, 4 mcg/2 mL
Rx Only
50 single-dose Vial in a Carton
AmnealPharmaceuticals LLC