Brand Name
Baxdela
Generic Name
Delafloxacin Meglumine
View Brand Information FDA approval date: June 19, 2017
Classification: Fluoroquinolone Antibacterial
Form: Injection, Tablet
What is Baxdela (Delafloxacin Meglumine)?
BAXDELA is a fluoroquinolone antibacterial indicated for the treatment of adults with the following infections caused by designated susceptible bacteria: Acute Bacterial Skin and Skin Structure Infections .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Baxdela (delafloxacin meglumine)
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS and EXACERBATION OF MYASTHENIA GRAVIS
Fluoroquinolones have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together (5.1), including:
- Tendinitis and tendon rupture (5.2)
- Peripheral neuropathy (5.3)
- Central nervous system effects (5.4)
Discontinue BAXDELA immediately and avoid the use of fluoroquinolones, including BAXDELA, in patients who experience any of these serious adverse reactions (5.1)
Fluoroquinolones may exacerbate muscle weakness in patients with myasthenia gravis. Avoid BAXDELA in patients with known history of myasthenia gravis. (5.5)
1CONTRAINDICATIONS
BAXDELA is contraindicated in patients with known hypersensitivity to delafloxacin or any of the fluoroquinolone class of antibacterial drugs, or any of the components of BAXDELA
2ADVERSE REACTIONS
The following serious and otherwise important adverse reactions are discussed in greater detail in other sections of labeling:
- Disabling and Potentially Irreversible Serious Adverse Reactions
- Tendinitis and Tendon Rupture
- Peripheral Neuropathy
- Central Nervous System Effects
- Hypersensitivity Reactions
- Clostridium difficile-Associated Diarrhea [see
- Blood Glucose Disturbances
2.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of BAXDELA cannot be directly compared to rates in the clinical trials of another drug and may not reflect rates observed in practice.
3OVERDOSAGE
Treatment of overdose with BAXDELA should consist of observation and general supportive measures. Hemodialysis removed about 19% of delafloxacin and 56% of SBECD (Sulfobutylether β cyclodextrin) after intravenous administration of BAXDELA
4DESCRIPTION
BAXDELA (delafloxacin) for Injection and BAXDELA (delafloxacin) Tablets contain meglumine salt of delafloxacin, a fluoroquinolone antibacterial. Delafloxacin meglumine is identified chemically as 1-Deoxy-1-(methylamino)-D-glucitol, 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (salt), the chemical structure of which is shown below. The meglumine salt has a molecular weight of 635.97 g/mol, whereas the molecular weight of the delafloxacin free acid is 440.76 g/mol.
Figure 1 Chemical Structure
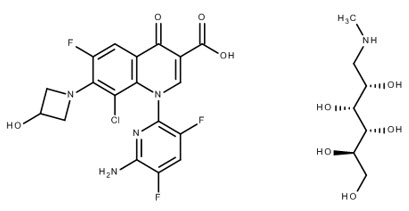
BAXDELA is intended for intravenous infusion or oral administration. BAXDELA is supplied as a sterile, lyophilized powder for injection and oral tablets as follows:
5PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA
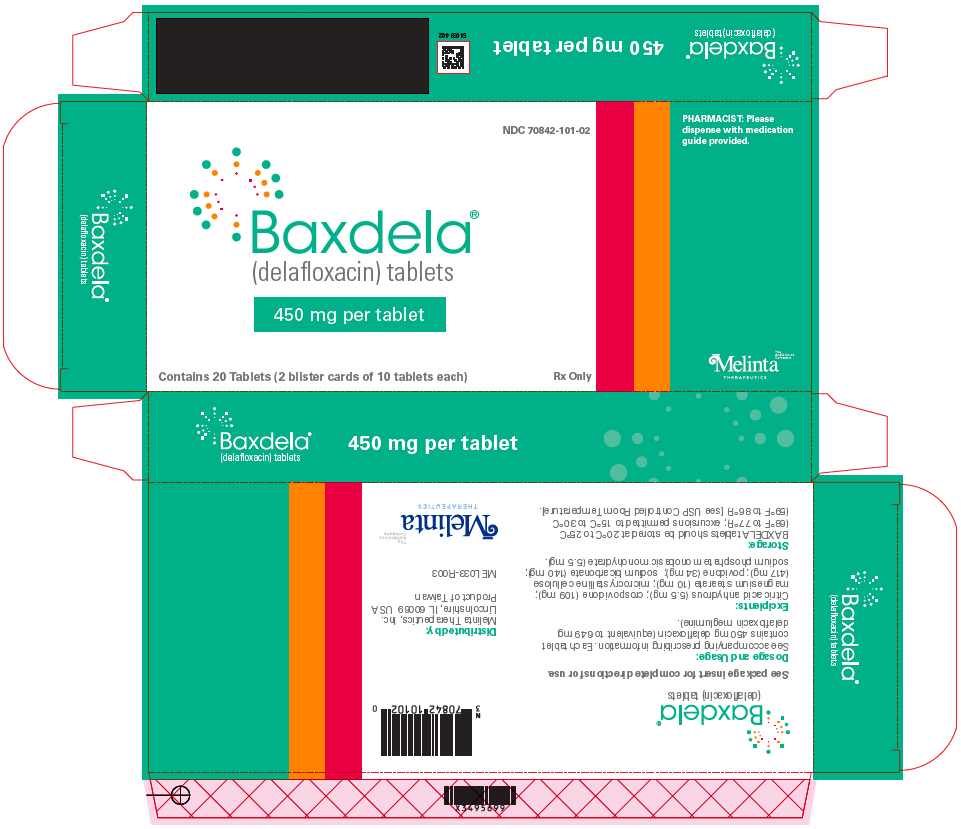
6PRINCIPAL DISPLAY PANEL - 450 mg Tablet Blister Pack Carton
NDC 70842-101-02
PHARMACIST: Please
Baxdela
450 mg per tablet
Contains 20 Tablets (2 blister cards of 10 tablets each)
Rx Only
Melinta
The
7PRINCIPAL DISPLAY PANEL - 450 mg Tablet Bottle Carton
NDC 70842-101-01
Baxdela
450 mg per tablet
Contains 20 Tablets
PHARMACIST: Please dispense
melinta

8PRINCIPAL DISPLAY PANEL - 20 Tablet Bottle Carton
NDC 70842-101-01
Baxdela
450 mg per tablet
Contains 20 Tablets
PHARMACIST: Please dispense
melinta

9PRINCIPAL DISPLAY PANEL - 300 mg Vial Carton
NDC 70842-102-03
Baxdela
300 mg per single-dose vial
Must be reconstituted and further diluted.
Contains 10 Vials
