Brand Name
Xadago
Generic Name
Safinamide
View Brand Information FDA approval date: May 08, 2017
Classification: Monoamine Oxidase Type B Inhibitor
Form: Tablet
What is Xadago (Safinamide)?
XADAGO is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson's disease experiencing "off" episodes. XADAGO is a monoamine oxidase type B inhibitor indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson's disease experiencing "off" episodes
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Prospective, Open Label, Single Arm, Clinical Study to Evaluate the Effect of Safinamide on Sleep Quality and Polysomnographic Parameters in Patients With Parkinson's Disease: the Safe Sleep Study
Summary: Patients suffering of Parkinson's Disease will be treated with 50 mg/day of Safinamide per os for 2 weeks (escalation phase). Then, safinamide will be increased up to 100 mg/day and, if tolerated, the treatment will be taken for 10 more weeks (maintenance phase). Total treatment 12 weeks.
Related Latest Advances
Brand Information
Xadago (safinamide mesylate)
1INDICATIONS AND USAGE
XADAGO is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson's disease (PD) experiencing "off" episodes.
2DOSAGE FORMS AND STRENGTHS
- 50 mg tablets: Orange to copper with metallic gloss, round, biconcave shaped embossed with "50" on one side
- 100 mg tablets: Orange to copper with metallic gloss, round, biconcave shaped embossed with "100" on one side
3CONTRAINDICATIONS
XADAGO is contraindicated in patients with:
- Concomitant use of other drugs in the monoamine oxidase inhibitor (MAOI) class or other drugs that are potent inhibitors of monoamine oxidase, including linezolid. The combination may result in increased blood pressure, including hypertensive crisis
- Concomitant use of opioid drugs (e.g., meperidine and its derivatives, methadone, propoxyphene, or tramadol); serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic, tetracyclic, or triazolopyridine antidepressants; cyclobenzaprine; methylphenidate, amphetamine, and their derivatives; or St John's wort. Concomitant use could result in life-threatening serotonin syndrome
- Concomitant use of dextromethorphan. The combination of MAOIs and dextromethorphan has been reported to cause episodes of psychosis or abnormal behavior
- A history of a hypersensitivity to safinamide. Reactions have included swelling of the tongue and oral mucosa, and dyspnea.
- Severe hepatic impairment (Child-Pugh C: 10-15)
4ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of labeling:
- Hypertension
- Serotonin Syndrome
- Falling Asleep During Activities of Daily Living
- Dyskinesia
- Hallucinations / Psychotic Behavior
- Impulse Control / Compulsive Behaviors
- Withdrawal-Emergent Hyperpyrexia and Confusion
- Retinal Pathology
4.1Clinical Trials Experience
Clinical trials are conducted under widely varying conditions; therefore, adverse reactions observed in the clinical trials of a drug cannot be directly compared to the incidence in the clinical trials of another drug and may not reflect the incidence observed in clinical practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of safinamide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Hypersensitivity: A postmarketing report describes a patient who developed a hypersensitivity reaction consisting of swelling of the tongue and gingiva, dyspnea and skin rash. The symptoms resolved shortly after XADAGO was discontinued, but reappeared following rechallenge a month later.
Nervous System Disorders: Headache
5OVERDOSAGE
There is no human experience with XADAGO overdose.
There is no known antidote to XADAGO nor any specific treatment for XADAGO overdose. If an overdose occurs, XADAGO treatment should be discontinued and supportive treatment should be administered as clinically indicated. In cases of overdose with XADAGO, dietary tyramine restriction should be observed for several weeks.
The Poison Control Center should be called at 1-800-222-1222 for the most current treatment guidelines.
6DESCRIPTION
XADAGO tablets contain safinamide, which is a MAO-B inhibitor, as the mesylate salt. Safinamide mesylate is (S)-2- [[4-[(3-fluorophenyl) methoxy]phenyl]methyl]aminopropanamide methanesulfonate (1:1) and its structural formula is below.
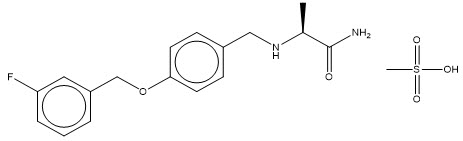
The molecular formula of safinamide mesylate is C
Safinamide mesylate is a white to off-white crystalline powder. Safinamide mesylate is freely soluble in water, methanol and dimethyl sulfoxide. Safinamide mesylate is sparingly soluble in ethanol and is practically insoluble in ethyl acetate. In aqueous buffers that span a pH range of 1.2 to 7.5, safinamide mesylate is highly soluble at pH 1.2 and 4.5, but shows low solubility (<0.4 mg/mL) at pH 6.8 and 7.5.
XADAGO is available as 50 mg and 100 mg film-coated tablets for oral administration. Each XADAGO tablet contains 65.88 mg or 131.76 mg of safinamide mesylate, equivalent to 50 mg or 100 mg, respectively, of safinamide free base. The tablets also contain the following inactive ingredients: colloidal silicon dioxide, crospovidone, hypromellose, iron oxide (red), magnesium stearate, microcrystalline cellulose, polyethylene glycol 6000, potassium aluminum silicate, and titanium dioxide.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
8PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle Carton
Rx only
Xadago
50 mg
Keep out of reach of children.
30 tablets
US WorldMeds™

9PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Carton
Rx only
Xadago
100 mg
Keep out of reach of children.
30 tablets
US WorldMeds™
