Endari
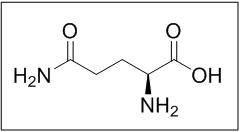
What is Endari (L-Glutamine)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Many veterans with Gulf War Illness developed chronic gastrointestinal symptoms during their deployment to the Persian Gulf. The pathophysiologic mechanisms of these chronic gastrointestinal symptoms are not well understood but cause significant morbidity in veterans. Our proposed studies will provide an innovative and novel treatment trial for chronic gastrointestinal symptoms in veterans with Gu...
Summary: The purpose of this study is to determine whether the combination of subcutaneous DRP-104 in combination with intravenous Durvalumab is safe and yields a clinically compelling antitumor activity measured as based on objective response rate (ORR, assessed by RECIST 1.1). Secondary objectives include progression-free survival (PFS) and overall survival (OS).
Summary: Metabolic reprogramming has been identified as a hallmark of cancer. Almost a century after Otto Warburg initially discovered increased glycolytic activity in tumor tissue (Warburg effect), therapeutic targeting of cancer metabolism has become a field of intense research effort in cancer biology. A growing appreciation of metabolic heterogeneity and complexity is currently reshaping investigators ...
Related Latest Advances
Brand Information
- Carton of 60 packets: NDC 42457-420-60
- Store Endari at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Endari away from direct sunlight.
(L-glutamine oral powder)


