Brand Name
Edarbi
Generic Name
Azilsartan kamedoxomil
View Brand Information FDA approval date: February 01, 2013
Classification: Angiotensin 2 Receptor Blocker
Form: Tablet
What is Edarbi (Azilsartan kamedoxomil)?
Edarbi is indicated for the treatment of hypertension to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with Edarbi. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure . Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension , and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects in black patients, and many antihypertensive drugs have additional approved indications and effects . These considerations may guide selection of therapy. Edarbi may be used alone or in combination with other antihypertensive agents. Edarbi is an angiotensin II receptor blocker indicated for the treatment of hypertension to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. Edarbi may be used either alone or in combination with other antihypertensive agents.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Edarbi (Azilsartan kamedoxomil)
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue Edarbi as soon as possible
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus
1INDICATIONS AND USAGE
Edarbi is indicated for the treatment of hypertension in adults, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with Edarbi.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Edarbi may be used alone or in combination with other antihypertensive agents.
2DOSAGE FORMS AND STRENGTHS
Edarbi is supplied as white to nearly white round tablets in the following dosage strengths:
- 40-mg tablets – debossed "ASL" on one side and "40" on the other
- 80-mg tablets – debossed "ASL" on one side and "80" on the other
3CONTRAINDICATIONS
Do not coadminister aliskiren-containing products with Edarbi in patients with diabetes
4OVERDOSAGE
Limited data are available related to overdosage in humans. During controlled clinical trials in healthy subjects, once-daily doses up to 320 mg of Edarbi were administered for seven days and were well tolerated. In the event of an overdose, supportive therapy should be instituted as dictated by the patient's clinical status. Azilsartan is not dialyzable
5DESCRIPTION
Edarbi (azilsartan medoxomil), a prodrug, is hydrolyzed to azilsartan in the gastrointestinal tract during absorption. Azilsartan is an angiotensin II receptor blocker.
The drug substance used in the drug product formulation is the potassium salt of azilsartan medoxomil, also known by the US accepted name of azilsartan kamedoxomil and is chemically described as (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1
Its empirical formula is C
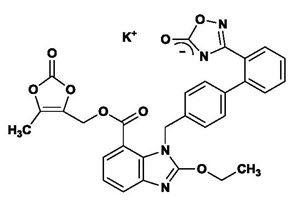
Azilsartan kamedoxomil is a white to nearly white powder with a molecular weight of 606.62. It is practically insoluble in water and freely soluble in methanol.
Edarbi is available for oral use as tablets. The tablets have a characteristic odor. Each Edarbi tablet contains 42.68 or 85.36 mg of azilsartan kamedoxomil, which is equivalent to containing 40 mg or 80 mg respectively, of azilsartan medoxomil and the following inactive ingredients: mannitol, fumaric acid, sodium hydroxide, hydroxypropyl cellulose, croscarmellose sodium, microcrystalline cellulose, and magnesium stearate.
6CLINICAL STUDIES
The antihypertensive effects of Edarbi have been demonstrated in a total of seven double-blind, randomized studies, which included five placebo-controlled and four active comparator-controlled studies (not mutually exclusive). The studies ranged from six weeks to six months in duration, at doses ranging from 20 mg to 80 mg once daily. A total of 5941 patients (3672 given Edarbi, 801 given placebo, and 1468 given active comparator) with mild, moderate or severe hypertension were studied. Overall, 51% of patients were male and 26% were 65 years or older; 67% were white and 19% were black.
Two 6-week, randomized, double-blind studies compared the effect on blood pressure of Edarbi at doses of 40 mg and 80 mg, with placebo and with active comparators. Blood pressure reductions compared to placebo based on clinic blood pressure measurements at trough and 24-hour mean blood pressure by ambulatory blood pressure monitoring (ABPM) are shown in Table 1 for both studies. Edarbi, 80 mg, was statistically superior to placebo and active comparators for both clinic and 24-hour mean blood pressure measurements.
In a study comparing Edarbi to valsartan over 24 weeks, similar results were observed.
Most of the antihypertensive effect occurs within the first two weeks of dosing.
Figure 2 shows the 24-hour ambulatory systolic and diastolic blood pressure profiles at endpoint.
Other studies showed similar 24-hour ambulatory blood pressure profiles.
Edarbi has a sustained and consistent antihypertensive effect during long-term treatment, as shown in a study that randomized patients to placebo or continued Edarbi after 26 weeks. No rebound effect was observed following the abrupt cessation of Edarbi therapy.
Edarbi was effective in reducing blood pressure regardless of the age, gender, or race of patients, but the effect, as monotherapy, was smaller, approximately half, in black patients, who tend to have low renin levels. This has been generally true for other angiotensin II antagonists and ACE inhibitors.
Edarbi has about its usual blood pressure lowering effect size when added to a calcium channel blocker (amlodipine) or a thiazide-type diuretic (chlorthalidone).
There are no trials of Edarbi demonstrating reductions in cardiovascular risk in patients with hypertension, but at least one pharmacologically similar drug has demonstrated such benefits.
7HOW SUPPLIED/STORAGE AND HANDLING
Edarbi tablets are unscored and white to nearly white, debossed with "ASL" on one side and "40" or "80" on the other.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information)
9PRINCIPAL DISPLAY PANEL - 40 mg Tablet Bottle Label
NDC 60631-040-30
edarbi
40 mg
Rx Only

10PRINCIPAL DISPLAY PANEL - 80 mg Tablet Bottle Label
NDC 60631-080-30
edarbi
80 mg
Rx Only
