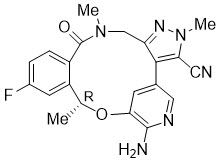
Lorbrena
What is Lorbrena (Lorlatinib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This phase III trial studies iobenguane I-131 or lorlatinib and standard therapy in treating younger patients with newly-diagnosed high-risk neuroblastoma or ganglioneuroblastoma. Radioactive drugs, such as iobenguane I-131, may carry radiation directly to tumor cells and not harm normal cells. Lorlatinib may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Gi...
Summary: Genes give your body instructions on how to make proteins. Proteins are needed to keep the body working properly. Many types of cancer are caused by changes in certain genes, making them faulty. Some people with non small cell lung cancer (NSCLC) have a faulty ALK gene. ALK stands for anaplastic lymphoma kinase. People with NSCLC who have the faulty ALK gene are called ALK-positive. ALK inhibitors...
Summary: The purpose of this study is to learn about lorlatinib for the possible treatment of lung cancer which could not be controlled. This study is seeking participants who: * have lung cancer that could not be controlled. * have a type of gene called a ROS proto-oncogene 1. A gene is a part of your DNA that has instructions for making things your body needs to work. * have received at least 1 treatment...
Related Latest Advances
Brand Information
- 25 mg: 8 mm round, tan, immediate release, film-coated, debossed with "Pfizer" on one side and "25" and "LLN" on the other side
- 100 mg: 8.5 mm × 17 mm oval, lavender, immediate release, film-coated, debossed with "Pfizer" on one side and "LLN 100" on the other side
- Risk of Serious Hepatotoxicity with Concomitant Use of Strong CYP3A Inducers
- Central Nervous System Effects
- Hyperlipidemia
- Atrioventricular Block
- Interstitial Lung Disease/Pneumonitis
- Hypertension
- Hyperglycemia