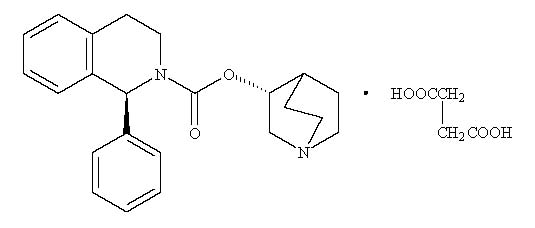
Solifenacin
What is VESIcare (Solifenacin)?
VESIcare (Solifenacin): Restoring Control for Overactive Bladder (OAB)
Living with an overactive bladder can be frustrating and isolating. The frequent, urgent need to urinate often with little warning can disrupt sleep, work, and social life. For many adults, this condition causes embarrassment and anxiety about leaving home or being far from a restroom. VESIcare (solifenacin) is a medication designed to bring back control and confidence by calming an overactive bladder and reducing those sudden urges.
VESIcare belongs to a class of drugs called **antimuscarinics**, which help relax the bladder muscles. Its a long-established, prescription-only treatment approved by the U.S. Food and Drug Administration (FDA) for managing overactive bladder (OAB) with symptoms such as urgency, frequency, and urge incontinence. Whether used as a first-line therapy or after other approaches like bladder training havent worked well enough, VESIcare is known for improving daily comfort and restoring quality of life.
What does VESIcare do?
VESIcare is used to treat the symptoms of overactive bladder, including:
- Urgency: a sudden, strong need to urinate immediately
- Frequency: needing to urinate too often, even during the night
- Urge incontinence: accidental leakage caused by inability to reach the bathroom in time
By calming the muscles that control urination, VESIcare helps the bladder hold urine longer and reduces unexpected leaks. Patients often notice fewer bathroom trips, less urgency, and improved sleep after consistent use.
Clinical studies have shown that solifenacin significantly reduces daily urinary frequency and incontinence episodes compared to placebo (Mayo Clinic, 2024). Many people begin noticing symptom relief within a few weeks, though full benefit may take longer as the body adjusts to the medication.
How does VESIcare work?
VESIcare works by blocking specific receptors in the bladder wall called **muscarinic receptors**. These receptors normally respond to acetylcholine, a natural body chemical that signals the bladder muscles to contract and release urine.
In people with overactive bladder, these signals can become overactive or misfired, causing the bladder to contract too often or at the wrong times. By blocking these receptors, solifenacin **reduces involuntary bladder contractions**, allowing the bladder to fill more completely before signaling the need to urinate.
Clinically, this mechanism matters because it restores bladder stability and capacity, giving patients more time between bathroom visits and greater control over their daily routines. Its a proven, safe way to manage bladder overactivity without requiring invasive procedures.
VESIcare Side Effects and Warnings
Like all prescription medicines, VESIcare can cause side effects. Most are mild and temporary, improving as the body adjusts to treatment.
Common Side Effects include:
- Dry mouth
- Constipation
- Blurred vision
- Mild drowsiness
- Dry eyes
These effects occur because solifenacin also reduces secretions and muscle activity in other parts of the body, not just the bladder. Drinking water regularly, using sugar-free gum for dry mouth, or adding fiber to the diet can help ease these symptoms.
Serious but less common side effects include:
- Painful or difficult urination (possible urinary retention)
- Confusion, hallucinations, or severe dizziness (more likely in older adults)
- Fast or irregular heartbeat
- Allergic reactions such as rash, swelling, or difficulty breathing
Who should avoid VESIcare:
People with **urinary retention**, uncontrolled narrow-angle glaucoma, severe liver disease, or certain gastrointestinal conditions like severe constipation or bowel obstruction should not use this medication. Those with kidney or liver impairment may need dose adjustments.
Seek immediate medical attention if you experience severe abdominal pain, vision changes, or an inability to urinate. Its also important to inform your doctor about all other medications you take, as VESIcare can interact with antifungal drugs, antibiotics, and certain heart medications.
VESIcare Dosage and Monitoring
VESIcare comes in tablet form, taken **once daily by mouth**, with or without food. Patients are advised to swallow the tablet whole with water, not to crush or chew it.
The dose is individualized based on symptom control, side effects, and overall health. Your healthcare provider may start with a lower dose and increase it if needed.
Because solifenacin is processed in the liver and kidneys, doctors may perform periodic tests to check organ function. Monitoring helps ensure safe and effective treatment, especially for older adults or those with preexisting medical conditions.
If a dose is missed, patients should take it as soon as they remember, unless its close to the next scheduled dose. Doubling up to make up for a missed dose is not recommended.
Does VESIcare have a generic version?
Yes. The generic form of VESIcare is solifenacin succinate, and it is FDA-approved. Generic solifenacin contains the same active ingredient, dosage strength, and effectiveness as the brand-name version.
Generic solifenacin, a more affordable alternative to VESIcare, offers the same safety and effectiveness. Patient preference for VESIcare may depend on availability, insurance, or individual response.
Conclusion
VESIcare (solifenacin) is a trusted, effective medication that helps people with overactive bladder regain control, confidence, and peace of mind. By relaxing bladder muscles and reducing involuntary contractions, it decreases the urgency and frequency of urination while preventing embarrassing leakage.
Although side effects like dry mouth or constipation can occur, they are typically manageable with simple lifestyle adjustments. Regular follow-up with a healthcare provider ensures that the medication remains safe and tailored to your needs.
Every patients experience is unique; what matters most is open communication with your doctor about any changes in symptoms or comfort level. When used correctly and monitored properly, VESIcare offers lasting relief, helping patients reclaim their independence and enjoy daily life without constant worry about bladder control.
References
- Mayo Clinic. (2024). Solifenacin (oral route) description and precautions. Retrieved from mayoclinic.org
- MedlinePlus. (2024). Solifenacin: Drug information. National Library of Medicine. Retrieved from medlineplus.gov
- U.S. Food and Drug Administration (FDA). (2023). Approved Drug Products: Solifenacin succinate. Retrieved from accessdata.fda.gov
- National Institutes of Health (NIH). (2024). Overactive bladder: Treatment and management. Retrieved from nih.gov
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- 5 mg: round, light yellow, debossed with 150
- 10 mg: round, light pink, debossed with 151
- With urinary retention
- With gastric retention
- With uncontrolled narrow-angle glaucoma
- Who have demonstrated hypersensitivity to solifenacin succinate or the inactive ingredients in VESIcare. Reported adverse reactions have included anaphylaxis and angioedema
- Bottle of 30 NDC 51248-150-01
- Bottle of 30 NDC 51248-151-01
- Bottle of 90 NDC 51248-151-03