Etopophos
What is Etopophos (Etoposide)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This phase II/III trial compares the effect of immunotherapy with atezolizumab in combination with standard chemotherapy with a platinum drug (cisplatin or carboplatin) and etoposide versus standard therapy alone for the treatment of poorly differentiated extrapulmonary (originated outside the lung) neuroendocrine cancer that may have spread from where it first started to nearby tissue, lymph node...
Background: High-grade neuroendocrine carcinomas (HGNEC) are cancers that develop in different parts of the body, including the digestive tract, genitals, neck, and head. One drug (belinostat), combined with 2 other drugs (etoposide and cisplatin), is approved to treat HGNEC. But some people may have a gene variant that affects how quickly their body gets rid of the drug; these people may do better with diffe...
Summary: This phase III trial tests how well the addition of dinutuximab to Induction chemotherapy along with standard of care surgical resection of the primary tumor, radiation, stem cell transplantation, and immunotherapy works for treating children with newly diagnosed high-risk neuroblastoma. Dinutuximab is a monoclonal antibody that binds to a molecule called GD2, which is found on the surface of neur...
Related Latest Advances
Brand Information
- Myelosuppression
- Secondary leukemias
- Hypersensitivity reactions
ETOPOPHOS has been used as a single agent in clinical studies involving 206 patients with a variety of malignancies (including one non-Hodgkin’s lymphoma) and in combination with cisplatin in 60 patients with small cell lung cancer. The most common adverse reaction was neutropenia.
Other Important Adverse Reactions
Gastrointestinal Toxicity
Nausea and vomiting are the major gastrointestinal toxicities. The severity of nausea and vomiting is generally mild to moderate, with treatment discontinuation required in 1% of patients. Nausea and vomiting are managed with standard antiemetic therapy.
Other clinically important adverse reactions in clinical trials were:
Gastrointestinal: abdominal pain, constipation, dysphagia
General: fever
Ocular: transient cortical blindness, optic neuritis
Respiratory: interstitial pneumonitis/pulmonary fibrosis
Skin: pigmentation, radiation recall dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis
Neurologic: seizure, aftertaste
Hepatobiliary disorder: hepatotoxicity
Extravasation, resulting in local soft tissue toxicity. Extravasation of ETOPOPHOS can result in swelling, pain, cellulitis, and necrosis, including skin necrosis.
Reversible cases of acute renal failure have been reported with administration of high dose (2220 mg/m2) ETOPOPHOS with total body irradiation used for hematopoietic stem cell transplantation. The ETOPOPHOS formulation contains dextran 40, which has been associated with acute renal failure when administered in high doses.
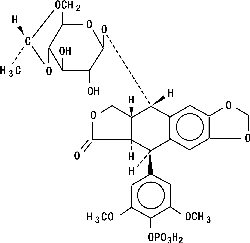
Etoposide phosphate has the following structure:
ETOPOPHOS is supplied as a single-dose vial containing etoposide phosphate equivalent to 100 mg etoposide as a lyophilized powder for reconstitution, individually packaged in a carton:
NDC 61269-410-20
Store unopened vials at 2° to 8°C (36°-46°F). Keep vial in outer carton to protect from light.
ETOPOPHOS is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
- Advise patients that periodic monitoring of their blood counts is required. Advise patients to contact their healthcare provider for new onset of bleeding, fever, or symptoms of infection
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy
- Advise females of reproductive potential to use effective contraception during and 6 months after treatment with ETOPOPHOS
- Advise males with female sexual partners of reproductive potential to use condoms during treatment with ETOPOPHOS and for at least 4 months after the final dose
Distributed by:
H2-Pharma, LLC
Montgomery, AL 36117


