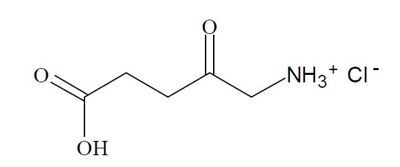
Aminolevulinic
What is Levulan Kerastick (Aminolevulinic)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This phase Ib trial tests the safety, best dose, and effectiveness of SONALA-001 in combination with magnetic resonance imaging-guided focused ultrasound (MRgFUS), also called sonodynamic therapy, in treating patients with glioblastoma that is growing, spreading, or getting worse (progressive) or that has come back after a period of improvement (recurrent). Sonodynamic therapy is a non-invasive co...
Summary: This Phase 3 study is designed to investigate the safety, diagnostic performance, and clinical usefulness of Gleolan for the real-time detection and visualization of epithelial ovarian cancer tumors during debulking surgery. The study is planned to run for about 18 months with individual study participation lasting about two (2) weeks.
Summary: Registry study to gather more information on the current use of Blue Light Cystoscopy with Cysview (BLCC) in urologists' practices.
Related Latest Advances
Brand Information
- Cutaneous photosensitivity at wavelengths of 400-450 nm
- Porphyria or known allergies to porphyrins
- Known sensitivity to any of the components of the LEVULAN KERASTICK.
- Transient Amnestic Episodes
- Increased Photosensitivity
- Irritation
- Coagulation defects
Inform patients that treatment with LEVULAN KERASTICK topical solution plus BLU-U Blue Light Photodynamic Therapy Illuminator may result in sensitivity to light, skin irritation and local skin reactions including erythema, edema, stinging/burning, scaling, crusting, oozing, vesiculation, wheal, scabbing, pustules, ulceration, itching, erosion, hypo/hyperpigmentation, bleeding, tenderness, dysesthesia, and dryness.
- Manufactured by: Sun Pharmaceutical Industries, Inc.
- Billerica, MA, 01821
- LAB-0530AW, Revision: F

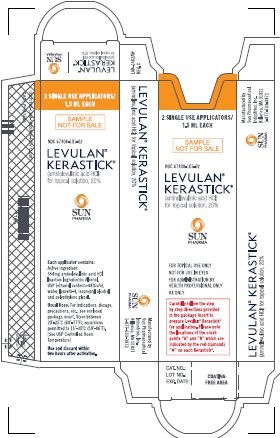
(aminolevulinic acid HCl) for Topical Solution, 20%
SINGLE USE APPLICATOR 1.5 mL
NDC 67308-101-01
A
↑
CRUSH
HERE
↓
A
LOT NO.:
EXP. DATE:
B
↑
CRUSH
HERE
↓
B
MANUFACTURED BY:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
LAB-0528 AW REV F

Carefully follow the step by step directions provided in the package insert to prepare Levulan ®Kerastick ®for application. Please note the locations of the crush points “A” and “B” which are indicated by the red diamonds “♦” on each Kerastick ®.
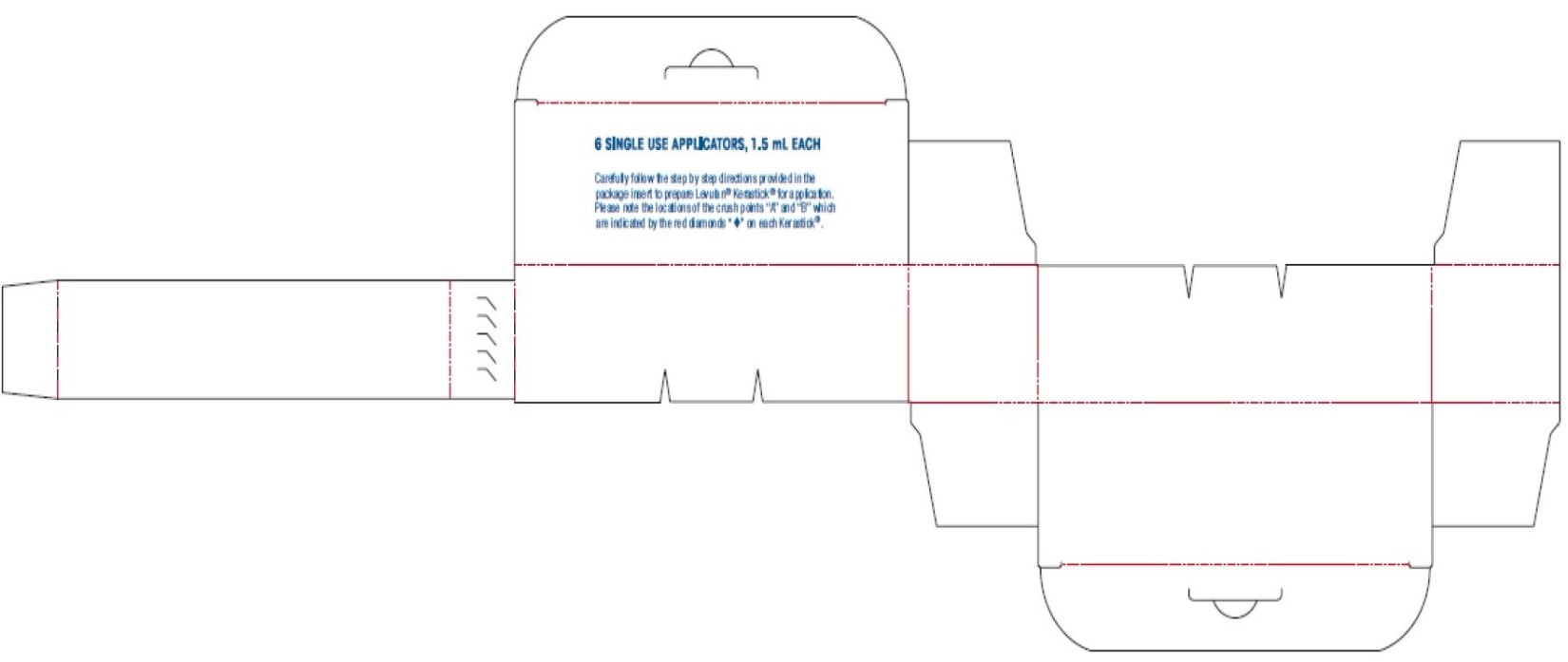

Levulan® Kerastick
(aminolevulinic acid HCl) for Topical Solution, 20%
2 SINGLE USE APPLICATORS, 1.5 mL EACH
SAMPLE
NOT FOR SALE
For Topical Use Only
Not For Use in Eyes
FOR ADMINISTRATION BY HEALTH PROFESSIONAL ONLY
Rx only
Carefully follow the step by step directions provided in the package to prepare Levulan ®Kerastick ®for application. Please note the locations of the crush points "A" and "B" which are indicated by the red diamonds "♦" on each Kerastick ®.
CAT. NO.:
LOT NO.:
EXP. DATE:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
1-877-533-3872
LAB-1462AW REV. C
Each applicator contains:
Active Ingredient: 354mg aminolevulinic acid HCl
Inactive Ingredients: Alcohol, USP (ethanol content-48% v/v), water, laureth-4, isopropyl alcohol, and polyethylene glycol.
Usual Dose:
For indications, dosage, precautions, etc., see enclosed package insert.
Store between 20°25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F)
[See USP Controlled Room Temperature]
Use and discard within two hours after activation.