Brand Name
Isradipine
View Brand InformationFDA approval date: January 05, 2004
Classification: Dihydropyridine Calcium Channel Blocker
Form: Capsule
What is Isradipine?
Hypertension Isradipine capsules are indicated in the management of hypertension. It may be used alone or concurrently with thiazide-type diuretics.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Isradipine (Isradipine)
1DESCRIPTION
Isradipine is a calcium antagonist available for oral administration in capsules containing 2.5 mg or 5 mg.
The structural formula of isradipine is:
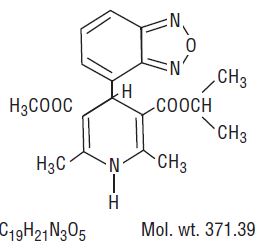
Chemically, isradipine is 3,5-Pyridinedicarboxylic acid, 4-(4-benzofurazanyl)-1,4-dihydro-2,6-dimethyl-, methyl 1-methylethyl ester. Isradipine is a yellow, fine crystalline powder which is odorless or has a faint characteristic odor. Isradipine is practically insoluble in water (<10 mg/L at 37ºC), but is soluble in ethanol and freely soluble in acetone, chloroform and methylene chloride.
Active Ingredient: isradipine
Inactive Ingredients: colloidal silicon dioxide, red iron oxide (2.5 mg capsule only), yellow iron oxide, gelatin, anhydrous lactose, magnesium stearate, sodium lauryl sulfate, starch (corn), titanium dioxide, black ink: black iron oxide, shellac, potassium hydroxide and propylene glycol.
2CONTRAINDICATIONS
Isradipine is contraindicated in individuals who have shown hypersensitivity to any of the ingredients in the formulation.
3WARNINGS
None
4ADVERSE REACTIONS
In multiple dose U.S. studies in hypertension, 1228 patients received isradipine alone or in combination with other agents, principally a thiazide diuretic, 934 of them in controlled comparisons with placebo or active agents. An additional 652 patients (which includes 374 normal volunteers) received isradipine in U.S. studies of conditions other than hypertension, and 1321 patients received isradipine in non-U.S. studies. About 500 patients received isradipine in long-term hypertension studies, 410 of them for at least 6 months. The adverse reaction rates given below are principally based on controlled hypertension studies, but rarer serious events are derived from all exposures to isradipine, including foreign marketing experience.
Most adverse reactions were mild and related to the vasodilatory effects of isradipine (dizziness, edema, palpitations, flushing, tachycardia), and many were transient. About 5% of isradipine patients left studies prematurely because of adverse reactions (vs. 3% of placebo patients and 6% of active control patients), principally due to headache, edema, dizziness, palpitations, and gastrointestinal disturbances.
The following table shows the most common adverse reactions, volunteered or elicited, considered by the investigator to be at least possibly drug related. The results for the isradipine treated patients are presented for all doses pooled together (reported by 1% or greater of patients receiving any dose of isradipine), and also for the two treatment regimens most applicable to the treatment of hypertension with isradipine: (1) initial and maintenance dose of 2.5 mg b.i.d., and (2) initial dose of 2.5 mg b.i.d. followed by maintenance dose of 5 mg b.i.d.
†Initial dose of 2.5 mg b.i.d. followed by maintenance dose of 5 mg b.i.d.
†† Initial dose of 2.5 mg b.i.d. followed by sequential titration to 5 mg b.i.d., 7.5 mg b.i.d., and maintenance dose of 10 mg b.i.d.
*Propranolol, prazosin, hydrochlorothiazide, enalapril, captopril.
Except for headache, which is not clearly drug-related (see previous table), the more frequent adverse reactions listed show little change, or increase slightly, in frequency over time, as shown in the following table:
Edema, palpitations, fatigue, and flushing appear to be dose-related, especially at the higher doses of 15 to 20 mg/day.
In open-label, long-term studies of up to two years in duration, the adverse events reported were generally the same as those reported in the short-term controlled trials. The overall frequencies of these adverse events were slightly higher in the long-term than in the controlled studies, but as in the controlled trials most adverse reactions were mild and transient.
The following adverse experiences were reported in 0.5% to 1.0% of the isradipine-treated patients in hypertension studies, or are rare. More serious events from this and other data sources, including postmarketing exposure, are shown in italics. The relationship of these adverse events to isradipine administration is uncertain.
Skin: pruritus, urticaria
Musculoskeletal: cramps of legs/feet
Respiratory: cough
Cardiovascular: shortness of breath, hypotension, atrial fibrillation, ventricular fibrillation, myocardial infarction, heart failure
Gastrointestinal: abdominal discomfort, constipation, diarrhea
Urogenital: nocturia
Nervous System: drowsiness, insomnia, lethargy, nervousness, impotence, decreased libido, depression, syncope, paresthesia (which includes numbness and tingling), transient ischemic attack, stroke
Autonomic: hyperhidrosis, visual disturbance, dry mouth, numbness
Miscellaneous: throat discomfort, leukopenia, elevated liver function tests
To report SUSPECTED ADVERSE EVENTS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch.
5OVERDOSAGE
Minimal empirical data are available on isradipine overdosage. Three individual suicide attempts with dosages of isradipine reported to be from 20 mg up to 100 mg resulted in lethargy, sinus tachycardia and, in the case of the person ingesting 100 mg, transient hypotension which responded to fluid therapy. A foreign report of the ingestion of 200 mg of isradipine with ethanol resulted only in flushing, tachycardia with ST depression on ECG, and hypotension, all of which were reversible. The ingestion of 5 mg of isradipine by a 22-month old child and the accidental ingestion of 100 mg of isradipine by a 58-year old female did not result in any sequelae.
Available data suggest that, as with other dihydropyridines, overdosage with isradipine might result in excessive peripheral vasodilatation with subsequent marked and probably prolonged systemic hypotension, and tachycardia. Emesis, gastric lavage, administration of activated charcoal followed in 30 minutes by a saline cathartic would be reasonable therapy. Isradipine is highly protein-bound and not removed by hemodialysis. Overdosage characterized by clinically significant hypotension should be treated with active cardiovascular support including monitoring of cardiac and respiratory function, elevation of lower extremities, and attention to circulating fluid volume and urine output. A vasoconstrictor (such as epinephrine, norepinephrine, or levarterenol) may be helpful in restoring a normotensive state, provided that there is no contraindication to its use.
Refractory hypotension or AV conduction disturbances may be treated with intravenous calcium salts, or glucagon. Cimetidine should be withheld in such instances due to the risk of further increasing plasma isradipine levels.
Significant lethality was observed in mice given oral doses of over 200 mg/kg and rabbits given about 50 mg/kg of isradipine. Rats tolerated doses of over 2000 mg/kg without effects on survival.
6DOSAGE AND ADMINISTRATION
The dosage of isradipine should be individualized. The recommended initial dose of isradipine is 2.5 mg b.i.d. alone or in combination with a thiazide diuretic. An antihypertensive response usually occurs within 2 to 3 hours. Maximal response may require 2 to 4 weeks. If a satisfactory reduction in blood pressure does not occur after this period, the dose may be adjusted in increments of 5 mg/day at 2 to 4 week intervals up to a maximum of 20 mg/day. Most patients, however, show no additional response to doses above 10 mg/day, and adverse effects are increased in frequency above 10 mg/day.
The bioavailability of isradipine (increased AUC) is increased in elderly patients (above 65 years of age), patients with hepatic functional impairment, and patients with mild renal impairment. Ordinarily, the starting dose should still be 2.5 mg b.i.d. in these patients.
7HOW SUPPLIED
Isradipine Capsules, USP
2.5 mgBrown opaque, imprinted with “ ” IS 2.5.
” IS 2.5.
Bottles of 100 capsules (NDC 16252-539-01)
 ” IS 2.5.
” IS 2.5.Bottles of 100 capsules (NDC 16252-539-01)
5 mgCaramel opaque, imprinted with “ ” IS 5.
” IS 5.
Bottles of 100 capsules (NDC 16252-540-01)
 ” IS 5.
” IS 5.Bottles of 100 capsules (NDC 16252-540-01)
Store and DispenseStore at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature] and dispense in a tight, light-resistant container.
Manufactured For:
Rev. A 11/2023
8PRINCIPAL DISPLAY PANEL
NDC 16252-539-01
Isradipine
100 Capsules

9PRINCIPAL DISPLAY PANEL
NDC 16252-540-01
Isradipine
Rx only
100 Capsules


