Brand Name
Meclofenamate
View Brand InformationFDA approval date: September 03, 1986
Form: Capsule
What is Meclofenamate?
Carefully consider the potential benefits and risks of meclofenamate sodium capsules and other treatment options before deciding to use meclofenamate sodium capsules. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals. Meclofenamate sodium capsules are indicated: For reduction of fever in adults, For relief of mild to moderate pain in adults, For relief of signs and symptoms of juvenile arthritis., For relief of the signs and symptoms of rheumatoid arthritis, For relief of the signs and symptoms of osteoarthritis., For treatment of primary dysmenorrhea., For acute or long-term use in the relief of signs and symptoms of the following: 1. Ankylosing spondylitis 2. Acute painful shoulder 3. Acute gouty arthritis Meclofenamate sodium capsules are also indicated for the treatment of idiopathic heavy menstrual blood loss. As with all nonsteroidal anti-inflammatory drugs, selection of meclofenamate sodium capsules require a careful assessment of the benefit/risk ratio. Meclofenamate sodium capsules are not recommended in children because adequate studies to demonstrate safety and efficacy have not been carried out.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Meclofenamate Sodium (meclofenamate sodium)
1DESCRIPTION
Meclofenamate sodium is N-(2,6-dichloro-m-tolyl) anthranilic acid, sodium salt, monohydrate. It is an anti-inflammatory drug for oral administration. Meclofenamate sodium capsules, USP contain 50 mg or 100 mg meclofenamic acid as the sodium salt and the following inactive ingredients: colloidal silicon dioxide, D&C Yellow No. 10, FD&C Blue No. 1, FD&C Red No. 3, gelatin, magnesium stearate, microcrystalline cellulose, pregelatinized starch (corn), sodium lauryl sulfate and titanium dioxide.
In addition, the imprinting ink contains black iron oxide, D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, propylene glycol and shellac glaze.
The structural formula of meclofenamate sodium is:
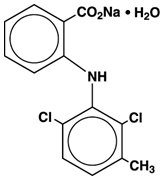
It is a white to creamy white, odorless to almost odorless, crystalline powder with melting point 287° to 291°C, molecular weight 336.15, and it is freely soluble in water.
2INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of meclofenamate sodium capsules and other treatment options before deciding to use meclofenamate sodium capsules. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see
Meclofenamate sodium capsules are indicated:
- For reduction of fever in adults
- For relief of mild to moderate pain in adults
- For relief of signs and symptoms of juvenile arthritis.
- For relief of the signs and symptoms of rheumatoid arthritis
- For relief of the signs and symptoms of osteoarthritis.
- For treatment of primary dysmenorrhea.
- For acute or long-term use in the relief of signs and symptoms of the following:
Meclofenamate sodium capsules are also indicated for the treatment of idiopathic heavy menstrual blood loss (see
As with all nonsteroidal anti-inflammatory drugs, selection of meclofenamate sodium capsules require a careful assessment of the benefit/risk ratio (see
Meclofenamate sodium capsules are not recommended in children because adequate studies to demonstrate safety and efficacy have not been carried out.
3CONTRAINDICATIONS
Meclofenamate sodium capsules are contraindicated in patients with known hypersensitivity to meclofenamate sodium.
Meclofenamate sodium capsules should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see
Meclofenamate sodium capsules are contraindicated in the setting of coronary artery bypass graft (CABG) surgery (see
4OVERDOSAGE
The following is based on the little information available concerning overdosage with meclofenamate sodium and related compounds. After a massive overdose, CNS stimulation may be manifested by irrational behavior, marked agitation and generalized seizures. Following this phase, renal toxicity (falling urine output, rising creatinine, abnormal urinary cellular elements) may be noted with possible oliguria or anuria and azotemia. A 24 year-old male was anuric for approximately one week after ingesting an overdose of 6 to 7 grams of meclofenamate sodium. Spontaneous diuresis and recovery subsequently occurred.
Management consists of emptying the stomach by emesis or lavage and instilling an ample dose of activated charcoal into the stomach. There is some evidence that charcoal will actively absorb meclofenamate sodium, but dialysis or hemoperfusion may be less effective because of plasma protein binding. The seizures should be controlled by an appropriate anticonvulsant regimen. Attention should be directed throughout, by careful monitoring, to the preservation of vital functions and fluid-electrolyte balance. Dialysis may be required to correct serious azotemia or electrolyte imbalance.
5DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of meclofenamate sodium capsules and other treatment options before deciding to use meclofenamate sodium capsules. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see
After observing the response to initial therapy with meclofenamate sodium capsules, the dose and frequency should be adjusted to suit an individual patient's needs.
6HOW SUPPLIED
Meclofenamate Sodium Capsules, USP are available containing either 50 mg or 100 mg of meclofenamic acid as the sodium salt.
The 50 mg capsules are hard-shell gelatin capsules with a coral opaque cap and a coral opaque body filled with an off-white powder blend. The capsules are axially printed with
NDC 0378-2150-01
The 100 mg capsules are hard-shell gelatin capsules with a coral opaque cap and a white opaque body filled with an off-white powder blend. The capsules are axially printed with
NDC 0378-3000-01
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light and moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
PHARMACIST: Dispense a Medication Guide with each prescription.
7Medication Guide for Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Manufactured by:
75107848
Revised: 12/2024
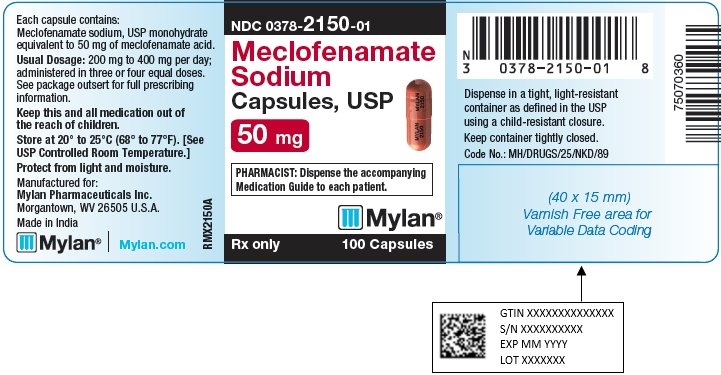
8PRINCIPAL DISPLAY PANEL – 50 mg
NDC 0378-2150-01
Meclofenamate
PHARMACIST: Dispense the accompanying
Rx only 100 Capsules
Each capsule contains:
Usual Dosage: 200 mg to 400 mg per day;
administered in three or four equal doses.
administered in three or four equal doses.
See package outsert for full prescribing
Keep this and all medication out of
Store at 20° to 25°C (68° to 77°F). [See
Protect from light and moisture.
Manufactured for:
Made in India
Mylan.com
RMX2150A1
Dispense in a tight, light-resistant
Keep container tightly closed.
Code No.: MH/DRUGS/25/NKD/89
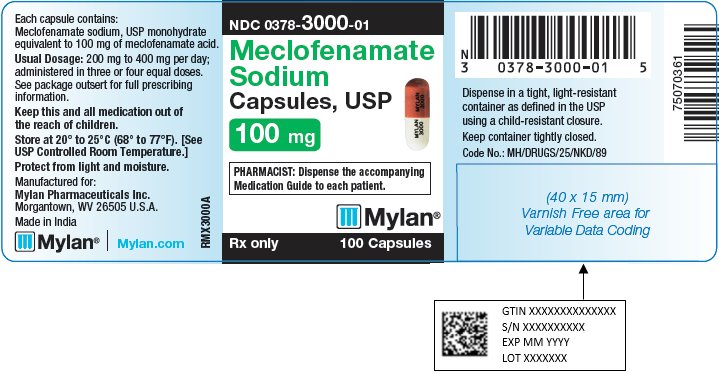
9PRINCIPAL DISPLAY PANEL – 100 mg
NDC 0378-3000-01
Meclofenamate
PHARMACIST: Dispense the accompanying
Rx only 100 Capsules
Each capsule contains:
Usual Dosage: 200 mg to 400 mg per day;
administered in three or four equal doses.
administered in three or four equal doses.
See package outsert for full prescribing
Keep this and all medication out of
Store at 20° to 25°C (68° to 77°F). [See
Protect from light and moisture.
Manufactured for:
Made in India
Mylan.com
RMX3000A1
Dispense in a tight, light-resistant
Keep container tightly closed.
Code No.: MH/DRUGS/25/NKD/89