Brand Name
Tracleer
Generic Name
Bosentan
View Brand Information FDA approval date: November 20, 2001
Classification: Endothelin Receptor Antagonist
Form: Tablet
What is Tracleer (Bosentan)?
Bosentan tablets are indicated for the treatment of pulmonary arterial hypertension : in adults to improve exercise ability and to decrease clinical worsening. Studies establishing effectiveness included predominantly patients with WHO Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH , PAH associated with connective tissue diseases , and PAH associated with congenital heart disease with left-to-right shunts [see Clinical Studies (1.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Tracleer (bosentan)
WARNING: RISKS OF HEPATOTOXICITY and EMBRYO-FETAL TOXICITY
Because of the risks of hepatotoxicity and birth defects, TRACLEER is available only through a restricted program called the Bosentan REMS Program. Under the Bosentan REMS, prescribers, patients, and pharmacies must enroll in the program
1INDICATIONS AND USAGE
TRACLEER is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1):
- in adults to improve exercise ability and to decrease clinical worsening. Studies establishing effectiveness included predominantly patients with WHO Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (60%), PAH associated with connective tissue diseases (21%), and PAH associated with congenital heart disease with left-to-right shunts (18%)
- in pediatric patients aged 3 years and older with idiopathic or congenital PAH to improve pulmonary vascular resistance (PVR), which is expected to result in an improvement in exercise ability.
2DOSAGE FORMS AND STRENGTHS
62.5 mg tablets: round, biconvex, orange-white tablets, debossed with identification marking "62,5"
125 mg tablets: oval, biconvex, orange-white tablets, debossed with identification marking "125"
32 mg tablets for oral suspension:
- quadrisected: clover-shaped, quadrisected, pale yellow to off-white tablets, debossed with identification marking "32" on the side opposite the quadrisection lines,
- bisected: round, pale yellow to off-white tablets, bisected on one side and debossed with identification marking "32" on the other side.
3ADVERSE REACTIONS
The following important adverse reactions are described elsewhere in the labeling:
- Hepatotoxicity
- Embryo-fetal Toxicity
- Fluid Retention
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Safety data on TRACLEER were obtained from 13 clinical studies (9 placebo-controlled and 4 open-label) in 870 adult patients with PAH and other diseases. Doses up to 8 times the currently recommended clinical dose (125 mg twice daily) were administered for a variety of durations. The exposure to TRACLEER in these trials ranged from 1 day to 4.1 years (n=94 for 1 year; n=61 for 1.5 years; and n=39 for more than 2 years). Exposure of PAH patients (n=328) to TRACLEER ranged from 1 day to 1.7 years (n=174 more than 6 months and n=28 more than 12 months).
Treatment discontinuations due to adverse events other than those related to pulmonary hypertension during the clinical trials in adult patients with PAH were more frequent on TRACLEER (6%; 15/258 patients) than on placebo (3%; 5/172 patients). In this database the only cause of discontinuations >1% and occurring more often on TRACLEER was abnormal liver function.
The adverse drug events that occurred in ≥3% of the TRACLEER-treated patients and were more common on TRACLEER in placebo-controlled trials in PAH at doses of 125 or 250 mg twice daily are shown in Table 3:
TRACLEER was evaluated for safety in 119 pediatric patients in uncontrolled studies. The safety profile was similar to that observed in adult patients with PAH.
3.2Postmarketing Experience
There have been several postmarketing reports of angioedema associated with the use of TRACLEER. The onset of the reported cases occurred within a range of 8 hours to 21 days after starting therapy. Some patients were treated with an antihistamine and their signs of angioedema resolved without discontinuing TRACLEER.
The following additional adverse reactions have been reported during the post approval use of TRACLEER. Because these adverse reactions are reported from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to TRACLEER exposure:
- Unexplained hepatic cirrhosis
- Liver failure
- Hypersensitivity, DRESS, and anaphylaxis
- Thrombocytopenia
- Rash
- Jaundice
- Anemia requiring transfusion
- Neutropenia and leukopenia
- Nasal congestion
- Autoimmune hepatitis
4OVERDOSAGE
Bosentan has been given as a single dose of up to 2400 mg in normal volunteers, or up to 2000 mg/day for 2 months in patients, without any major clinical consequences. The most common side effect was headache of mild to moderate intensity. In the cyclosporine A interaction study, in which doses of 500 and 1000 mg twice daily of bosentan were given concomitantly with cyclosporine A, trough plasma concentrations of bosentan increased 30-fold, resulting in severe headache, nausea, and vomiting, but no serious adverse events. Mild decreases in blood pressure and increases in heart rate were observed.
In the postmarketing period, there was one reported overdose of 10,000 mg of TRACLEER taken by an adolescent male patient. He had symptoms of nausea, vomiting, hypotension, dizziness, sweating, and blurred vision. He recovered within 24 hours with blood pressure support.
Bosentan is unlikely to be effectively removed by dialysis due to the high molecular weight and extensive plasma protein binding.
5DESCRIPTION
TRACLEER
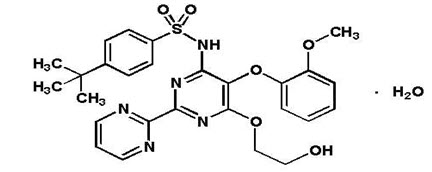
Bosentan has a molecular weight of 569.64 and a molecular formula of C
TRACLEER is available as 62.5 mg and 125 mg film-coated tablets for oral administration, and contains the following excipients: corn starch, ethylcellulose, glyceryl behenate, hydroxypropylmethylcellulose, iron oxide red, iron oxide yellow, magnesium stearate, povidone, pregelatinized starch, sodium starch glycolate, talc, titanium dioxide, and triacetin. Each TRACLEER 62.5 mg tablet contains 64.54 mg of bosentan monohydrate, equivalent to 62.5 mg of anhydrous bosentan. Each TRACLEER 125 mg tablet contains 129.08 mg of bosentan monohydrate, equivalent to 125 mg of anhydrous bosentan.
TRACLEER is also available as a 32 mg tablet for oral suspension and contains the following excipients: acesulfame potassium, aspartame (E951), calcium hydrogen phosphate anhydrous, cellulose microcrystalline, croscarmellose sodium, magnesium stearate, silica colloidal anhydrous, tartaric acid, and tutti frutti flavor. Each dispersible tablet contains 1.87 mg of phenylalanine. Each dispersible tablet contains 33.045 mg of bosentan monohydrate, equivalent to 32 mg anhydrous bosentan.
6HOW SUPPLIED/STORAGE AND HANDLING
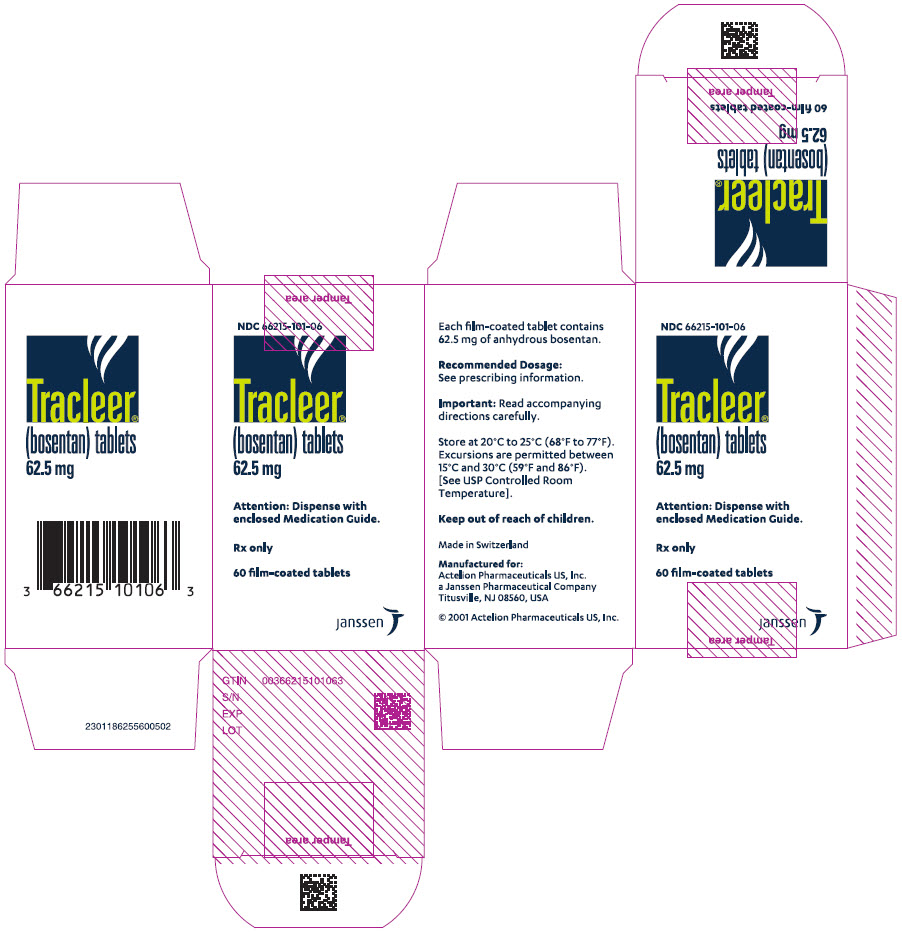
62.5 mgfilm-coated, round, biconvex, orange-white tablets, debossed with identification marking "62,5", packaged in a white high-density polyethylene bottle and a white polypropylene child-resistant cap or in foil blister-strips for hospital unit-dosing.
NDC 66215-101-06: Bottle containing 60 tablets.
NDC 66215-101-03: Carton of 30 tablets in 10 blister strips of 3 tablets.
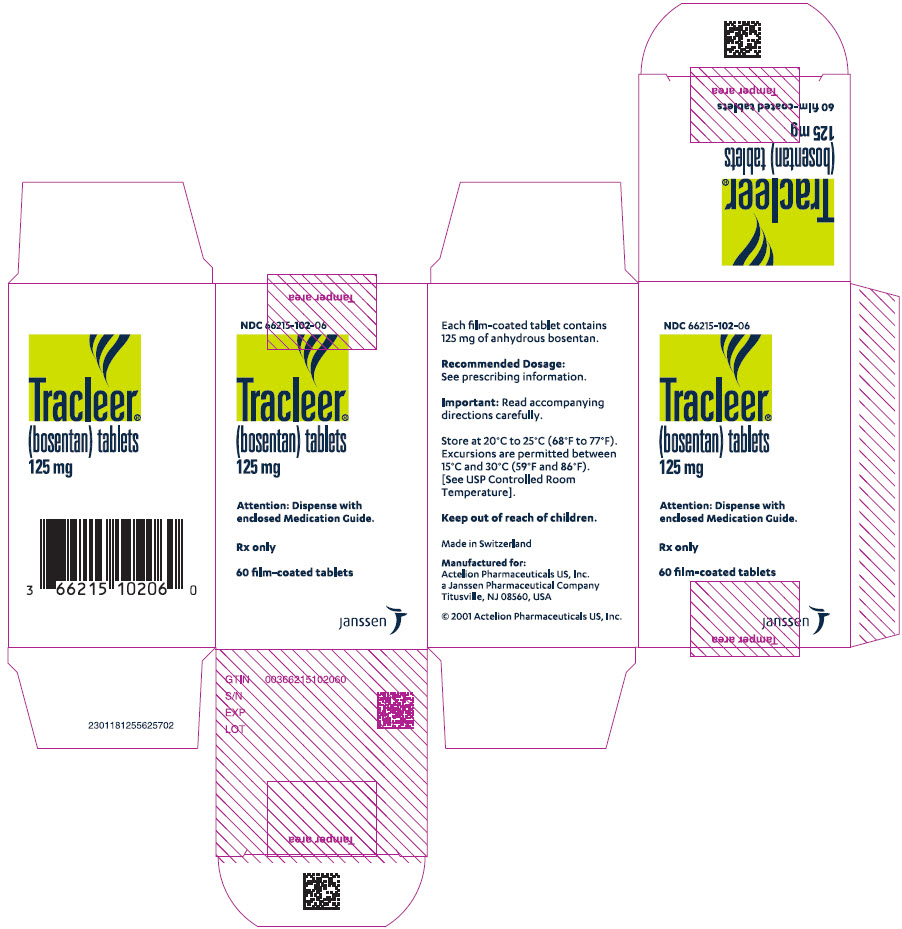
125 mgfilm-coated, oval, biconvex, orange-white tablets, debossed with identification marking "125", packaged in a white high-density polyethylene bottle and a white polypropylene child-resistant cap or in foil blister-strips for hospital unit-dosing.
NDC 66215-102-06: Bottle containing 60 tablets.
NDC 66215-102-03: Carton of 30 tablets in 10 blister strips of 3 tablets.
32 mgtablets for oral suspension, pale yellow to off-white, clover-shaped, quadrisected on one side and debossed with identification marking "32" on the other side, packaged in child resistant Aluminum/Aluminum peel-push blisters.
NDC 66215-103-56: Carton of 56 tablets for oral suspension in 4 blister-strips of 14 tablets.
NDC 66215-103-14: Blister strip of 14 tablets for oral suspension.
32 mgtablets for oral suspension, pale yellow to off-white, round, bisected on one side and debossed with identification marking "32" on the other side, packaged in child resistant Aluminum/Aluminum peel-push blisters.
NDC 66215-232-56: Carton of 56 tablets for oral suspension in 4 blister-strips of 14 tablets.
NDC 66215-232-14: Blister strip of 14 tablets for oral suspension.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
8PRINCIPAL DISPLAY PANEL - 62.5 mg Tablet Bottle Carton
NDC 66215-101-06
Tracleer
Attention: Dispense with
Rx only
60 film-coated tablets
janssen
9PRINCIPAL DISPLAY PANEL - 125 mg Tablet Bottle Carton
NDC 66215-102-06
Tracleer
Attention: Dispense with
Rx only
60 film-coated tablets
janssen
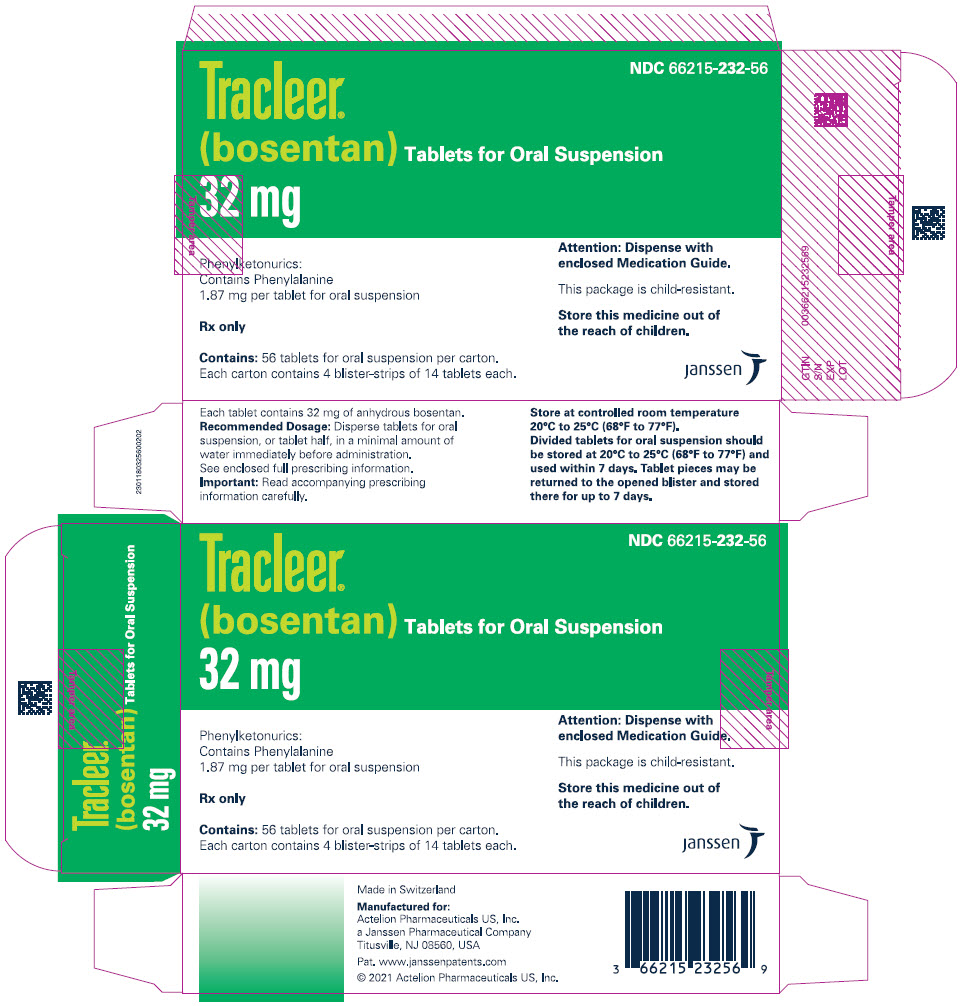
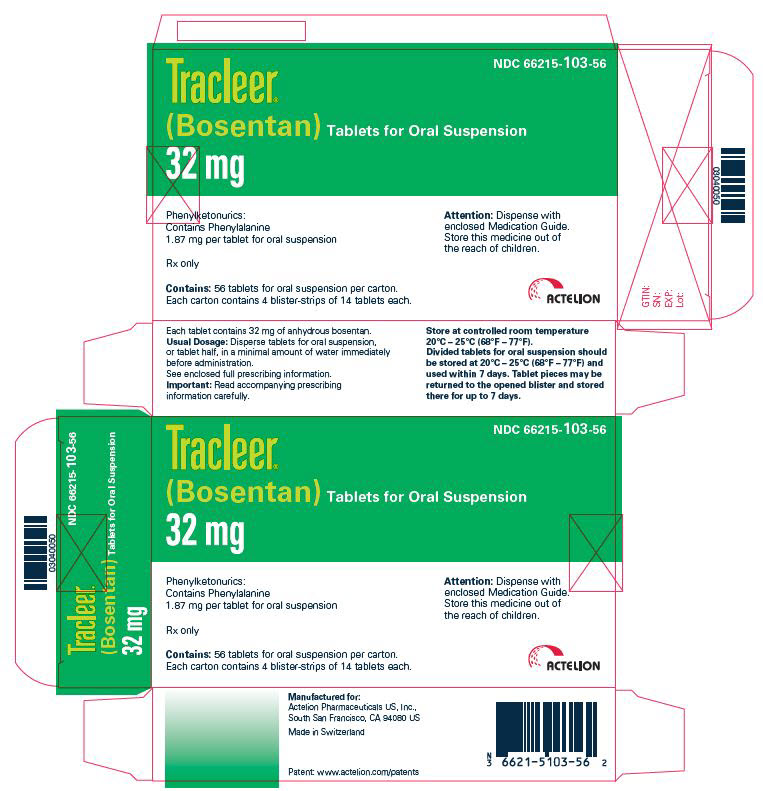
10PRINCIPAL DISPLAY PANEL - 32 mg Tablet Blister Pack Carton
NDC 66215-103-56
TRACLEER Tablets for Oral Suspension
32 mg
Phenylketonurics:
Rx only
Contains:56 tablets for oral suspension per carton.
Each carton contains 4 blister-strips of 14 tablets each.
Each carton contains 4 blister-strips of 14 tablets each.
Attention:Dispense with
enclosed Medication Guide.
Store this medicine out of
the reach of children.
enclosed Medication Guide.
Store this medicine out of
the reach of children.
ACTELION
11PRINCIPAL DISPLAY PANEL - 32 mg Tablet Blister Pack Carton - 232
NDC 66215-232-56
Tracleer
32 mg
Phenylketonurics:
Rx only
Contains: 56 tablets for oral suspension per carton.
Attention: Dispense with
This package is child-resistant.
Store this medicine out of
janssen