Brand Name
Hycamtin
Generic Name
Topotecan
View Brand Information FDA approval date: November 30, 2010
Classification: Topoisomerase Inhibitor
Form: Injection, Capsule
What is Hycamtin (Topotecan)?
HYCAMTIN ® capsules are indicated for the treatment of relapsed small cell lung cancer in patients with a prior complete or partial response and who are at least 45 days from the end of first-line chemotherapy. HYCAMTIN capsules is a topoisomerase inhibitor indicated for treatment of patients with relapsed small cell lung cancer .
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
HYCAMTIN (topotecan)
WARNING: MYELOSUPPRESSION
HYCAMTIN can cause severe myelosuppression. Administer first cycle only to patients with baseline neutrophil counts of greater than or equal to 1,500/mm
1INDICATIONS AND USAGE
HYCAMTIN
2DOSAGE FORMS AND STRENGTHS
Capsules
- 0.25 mg: opaque white to yellowish-white and imprinted with HYCAMTIN and 0.25 mg.
- 1 mg: opaque pink and imprinted with HYCAMTIN and 1 mg.
3CONTRAINDICATIONS
HYCAMTIN is contraindicated in patients who have a history of severe hypersensitivity reactions to topotecan. Reactions have included anaphylactoid reactions
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Myelosuppression
- Diarrhea
- Interstitial Lung Disease (ILD)
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the Warnings and Precautions and below reflects exposure to HYCAMTIN capsules in 682 patients with recurrent lung cancer enrolled in four randomized, open label trials, including 275 patients with small lung cell lung cancer (SCLC) (Studies 478, 065 and 396), and 407 patients with non-small cell lung cancer (NSCLC) (Study 387), who received at least one dose of HYCAMTIN capsules. Patients in these trials had advanced lung cancer and received prior chemotherapy in the first-line setting. Patients received HYCAMTIN capsules 2.3 mg/m
The safety of HYCAMTIN capsules was evaluated in a randomized trial (Study 478) conducted in 70 patients with recurrent SCLC
In the 682 patients who received HYCAMTIN capsules in the four lung cancer trials, 39 deaths (6%) occurred within 30 days after the last dose for a reason other than progressive disease: 13 due to hematologic toxicity, 5 due to non-hematologic toxicity (2 from diarrhea), and 21 due to other causes.
Table 1 describes the hematologic and non-hematologic adverse reactions that occurred in greater than 5% of patients treated with HYCAMTIN capsules in these trials.
4.2Postmarketing Experience
The following reactions have been identified during post approval use of HYCAMTIN. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal: Gastrointestinal perforation
General and Administration Site Conditions: Mucosal inflammation
Hypersensitivity: Allergic manifestations, anaphylactoid reactions, angioedema
5OVERDOSAGE
Overdoses (up to 5-fold of the prescribed dose) have occurred in patients receiving HYCAMTIN capsules. The primary complication of overdosage is myelosuppression. Mucositis have occurred with overdosages. If an overdose is suspected, monitor the patient closely for myelosuppression and institute supportive-care measures as appropriate.
6DESCRIPTION
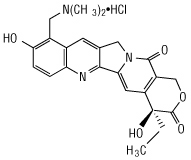
Topotecan is a topoisomerase inhibitor. The chemical name for topotecan hydrochloride is (
Topotecan hydrochloride has the following structural formula:
HYCAMTIN capsules, contain topotecan hydrochloride, the content of which is expressed as topotecan free base. Each 0.25 mg and 1 mg capsule contain topotecan hydrochloride equivalent to 0.25 mg and 1 mg topotecan free-base, respectively. The excipients are gelatin, glyceryl monostearate, hydrogenated vegetable oil, and titanium dioxide. The capsules are imprinted with edible black ink. The 1 mg capsules also contain red iron oxide.
7REFERENCES
- “OSHA Hazardous Drugs.”
8HOW SUPPLIED/STORAGE AND HANDLING
The 0.25 mg HYCAMTIN capsules are opaque white to yellowish-white imprinted with HYCAMTIN and 0.25 mg and are available in bottles of 10: NDC 0078-0672-01.
The 1 mg HYCAMTIN capsules are opaque pink imprinted with HYCAMTIN and 1 mg and are available in bottles of 10: NDC 0078-0673-01.
Store refrigerated 2ºC to 8ºC (36ºF to 46ºF) protected from light in the original carton.
HYCAMTIN is a cytotoxic drug. Follow applicable special handling and disposable procedures.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myelosuppression
Inform patients that HYCAMTIN decreases blood cell counts, such as white blood cells, platelets, and red blood cells. Instruct patients to notify their healthcare provider promptly for fever or other signs of infection
Diarrhea
Inform patients that HYCAMTIN capsules can cause diarrhea which may be severe and life-threatening. Instruct patients how to manage and/or prevent diarrhea and to inform their physician if severe diarrhea occurs during treatment with HYCAMTIN capsules
Interstitial Lung Disease (ILD)
Inform patients of the risks of severe ILD. Advise patients to contact their healthcare provider immediately to report new or worsening respiratory symptoms
Embryo-Fetal Toxicity
Advise females of reproductive potential and males with female partners of reproductive potential of the potential risk to a fetus. Advise women to contact their healthcare provider if they become pregnant, or if pregnancy is suspected during treatment with HYCAMTIN capsules
Advise females of reproductive potential to use effective contraception during treatment and for 6 months after the last dose of HYCAMTIN capsules
Advise males with a female partner of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of HYCAMTIN capsules
Lactation
Advise women to discontinue breastfeeding during treatment and for 1 week after the last dose of HYCAMTIN capsules
Infertility
Advise male and female patients of the potential risk for impaired fertility
Distributed by:
© Novartis
T2018-112