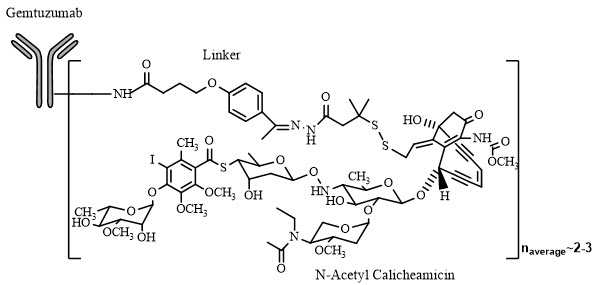
Mylotarg
What is Mylotarg (Gemtuzumab Ozogamicin)?
Approved To Treat
Related Clinical Trials
Summary: This phase II trial studies how well tretinoin and arsenic trioxide with or without gemtuzumab ozogamicin works in treating patients with previously untreated acute promyelocytic leukemia. Drugs used in chemotherapy, such as tretinoin and arsenic trioxide, work in different ways to stop the growth of cancer cells, either by killing the cells, by stopping them from dividing, or by stopping them fro...
Summary: This MyeloMATCH Master Screening and Reassessment Protocol (MSRP) evaluates the use of a screening tool and specific laboratory tests to help improve participants' ability to register to clinical trials throughout the course of their myeloid cancer (acute myeloid leukemia or myelodysplastic syndrome) treatment. This study involves testing patients' bone marrow and blood for certain biomarkers. A b...
Summary: A study to evaluate if the randomized addition of venetoclax to a chemotherapy backbone (fludarabine/cytarabine/gemtuzumab ozogamicin \[GO\]) improves survival of children/adolescents/young adults with acute myeloid leukemia (AML) in 1st relapse who are unable to receive additional anthracyclines, or in 2nd relapse.
Related Latest Advances
Brand Information
- Hepatotoxicity, including VOD
- Infusion-related reactions
- Hemorrhage