Temodar
What is Temodar (Temozolomide)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is a 2-part study. The purpose of Part 1 of the study is to evaluate the efficacy, safety, and pharmacokinetic (PK) characteristics of safusidenib in participants with recurrent/progressive IDH1-mutant World Health Organization (WHO) Grade 2 or Grade 3 glioma. The purpose of Part 2 will be to evaluate the efficacy of maintenance safusidenib treatment versus placebo in IDH1-mutant Grade 3 astr...
Summary: This phase III trial tests how well the addition of dinutuximab to Induction chemotherapy along with standard of care surgical resection of the primary tumor, radiation, stem cell transplantation, and immunotherapy works for treating children with newly diagnosed high-risk neuroblastoma. Dinutuximab is a monoclonal antibody that binds to a molecule called GD2, which is found on the surface of neur...
Summary: The researchers are doing this study to find out whether the drugs ABBV-637 and ABBV-155 are safe treatments that cause few or mild side effects when given alone or in combination with ERAS-801 in people with recurrent GBM.
Related Latest Advances
Brand Information
- Capsules:
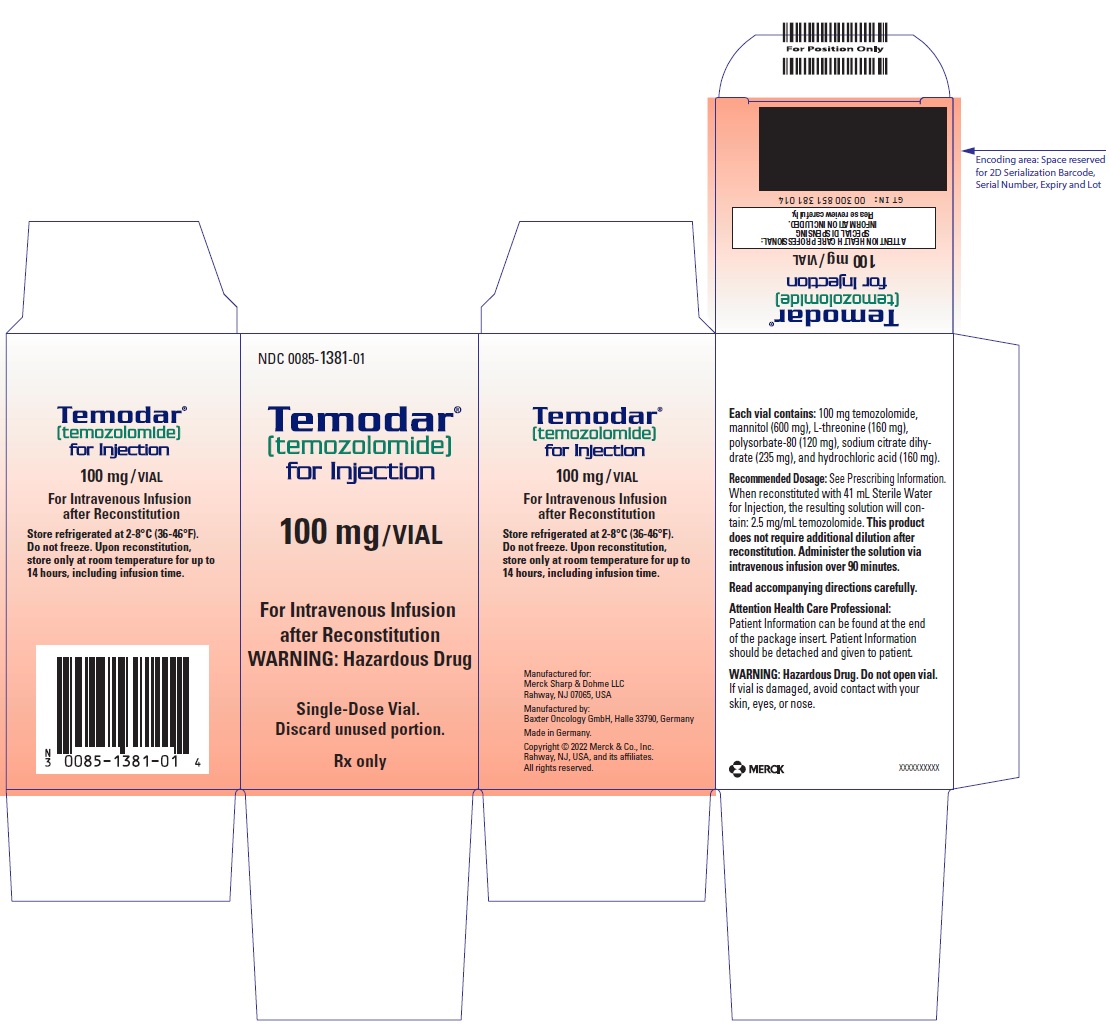
- For injection: 100 mg white to light tan or light pink lyophilized powder for reconstitution in a single-dose vial.
- temozolomide or any other ingredients in TEMODAR; and
- dacarbazine, since both temozolomide and dacarbazine are metabolized to the same active metabolite 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide.
- Myelosuppression
- Hepatotoxicity
- Pneumocystis Pneumonia [see
- Secondary Malignancies
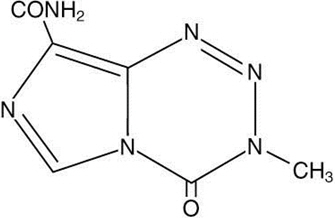
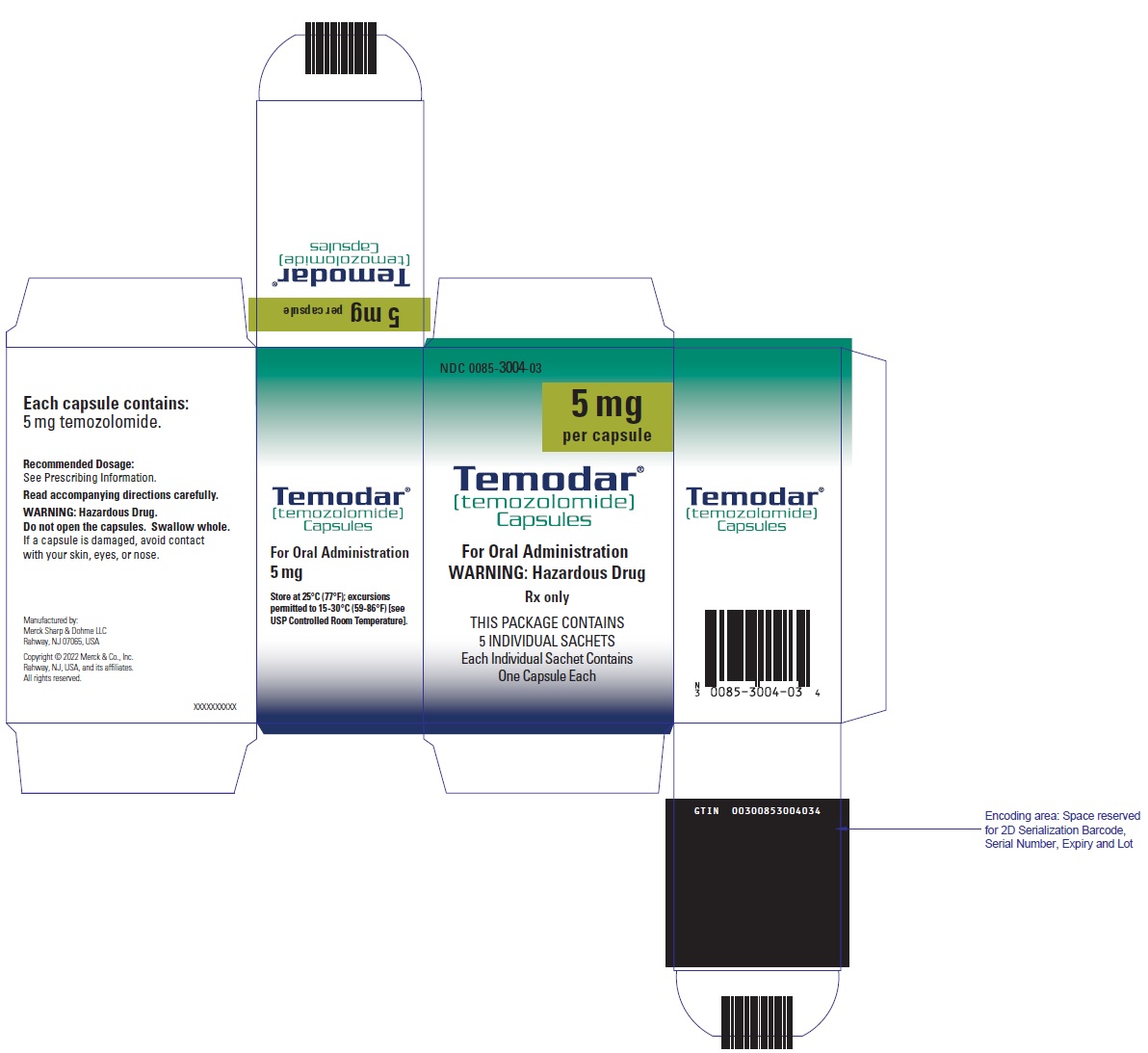
- TEMODAR 5 mg: lactose anhydrous (132.8 mg), colloidal silicon dioxide (0.2 mg), sodium starch glycolate (7.5 mg), tartaric acid (1.5 mg), and stearic acid (3 mg).
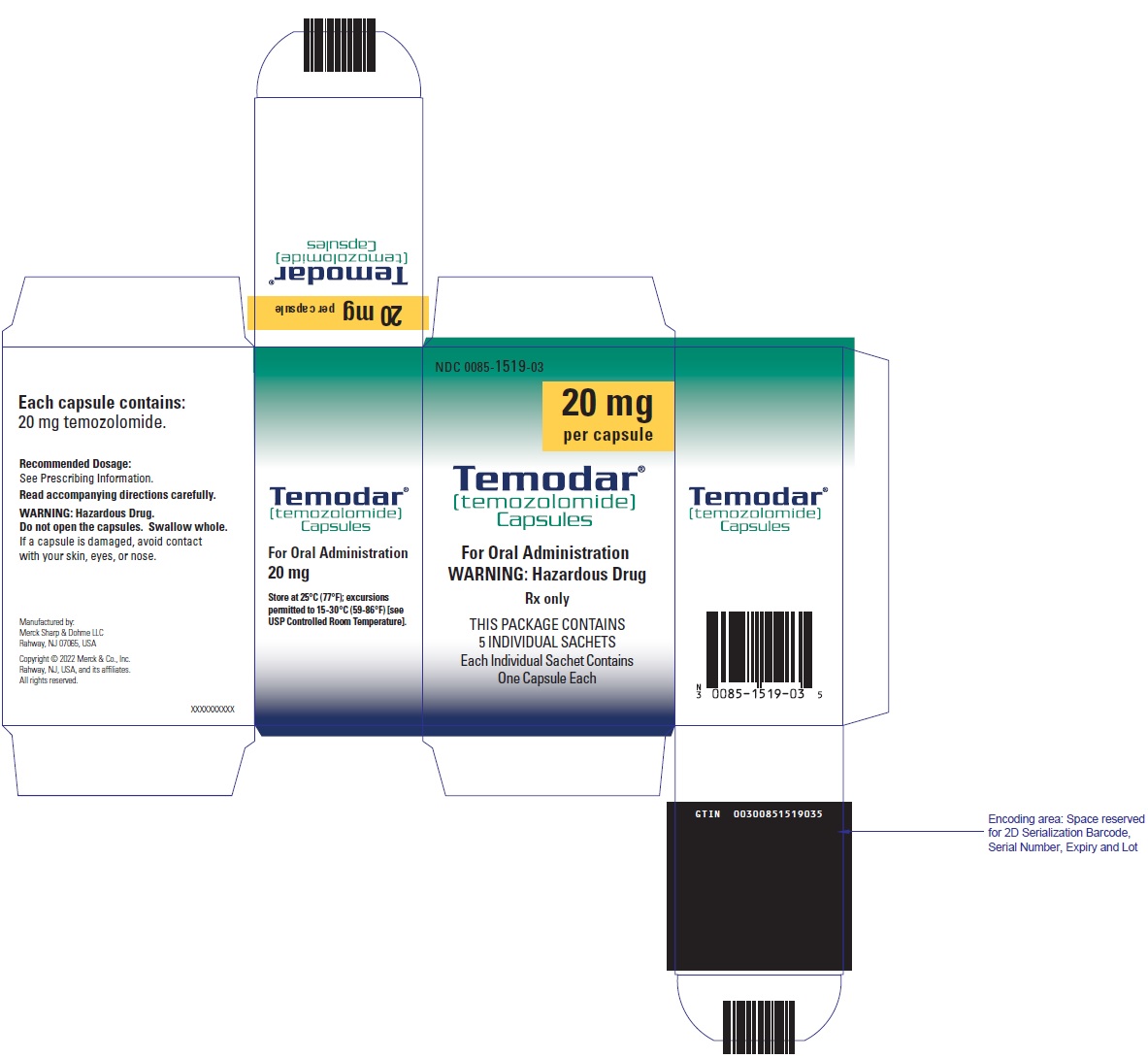
- TEMODAR 20 mg: lactose anhydrous (182.2 mg), colloidal silicon dioxide (0.2 mg), sodium starch glycolate (11 mg), tartaric acid (2.2 mg), and stearic acid (4.4 mg).
- TEMODAR 100 mg: lactose anhydrous (175.7 mg), colloidal silicon dioxide (0.3 mg), sodium starch glycolate (15 mg), tartaric acid (3 mg), and stearic acid (6 mg).
- TEMODAR 140 mg: lactose anhydrous (246 mg), colloidal silicon dioxide (0.4 mg), sodium starch glycolate (21 mg), tartaric acid (4.2 mg), and stearic acid (8.4 mg).
- TEMODAR 180 mg: lactose anhydrous (316.3 mg), colloidal silicon dioxide (0.5 mg), sodium starch glycolate (27 mg), tartaric acid (5.4 mg), and stearic acid (10.8 mg).
- TEMODAR 250 mg: lactose anhydrous (154.3 mg), colloidal silicon dioxide (0.7 mg), sodium starch glycolate (22.5 mg), tartaric acid (9 mg), and stearic acid (13.5 mg).
- TEMODAR 5 mg: The green cap contains gelatin, titanium dioxide, iron oxide yellow, sodium lauryl sulfate, and FD&C Blue #2.
- TEMODAR 20 mg: The yellow cap contains gelatin, sodium lauryl sulfate, and iron oxide yellow.
- TEMODAR 100 mg: The pink cap contains gelatin, titanium dioxide, sodium lauryl sulfate, and iron oxide red.
- TEMODAR 140 mg: The blue cap contains gelatin, sodium lauryl sulfate, and FD&C Blue #2.
- TEMODAR 180 mg: The orange cap contains gelatin, iron oxide red, iron oxide yellow, titanium dioxide, and sodium lauryl sulfate.
- TEMODAR 250 mg: The white cap contains gelatin, titanium dioxide, and sodium lauryl sulfate.
- “OSHA Hazardous Drugs.” OSHA.
[temozolomide]
Capsules
[temozolomide]
Capsules
[temozolomide]
Capsules
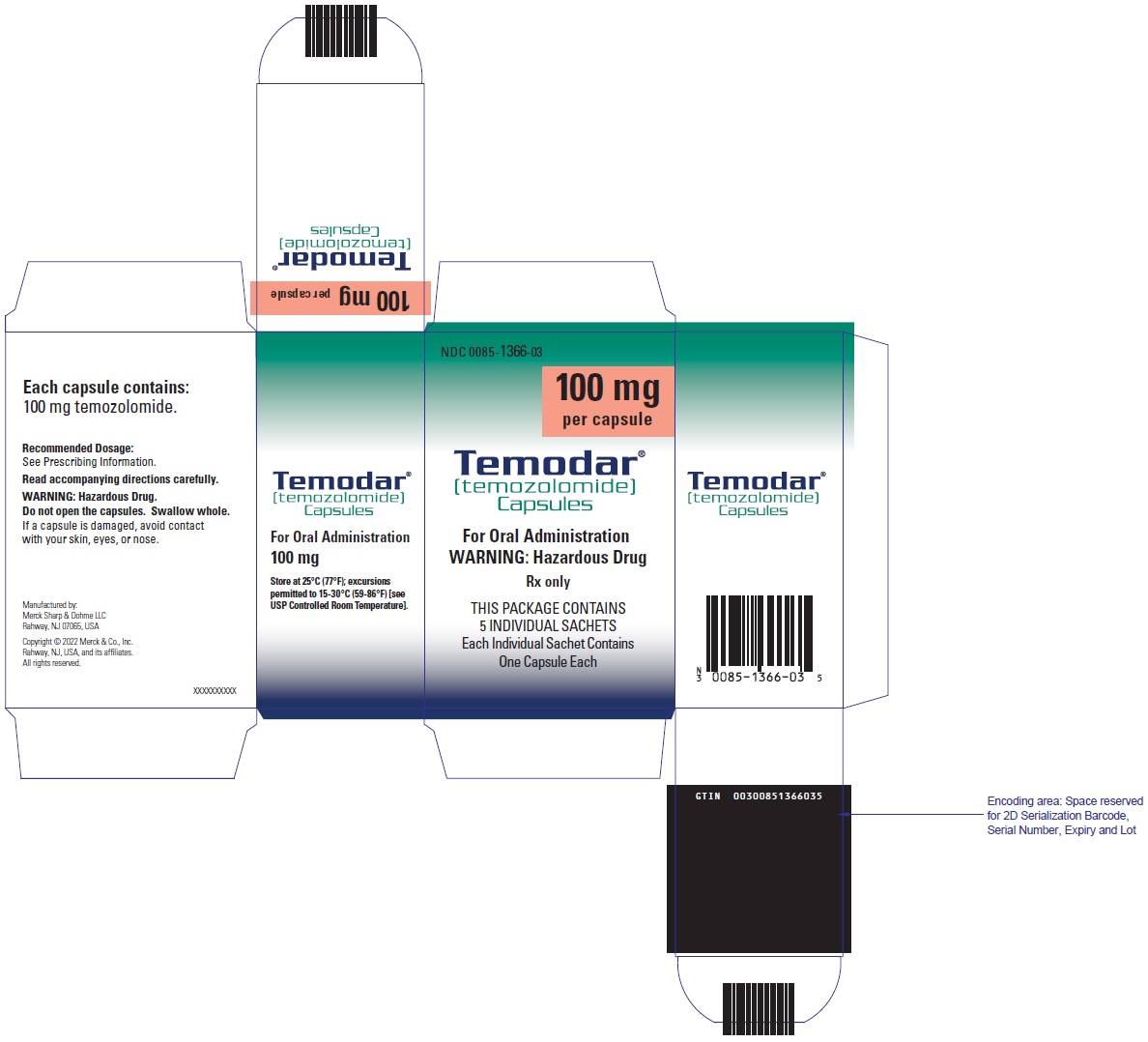
[temozolomide]
Capsules
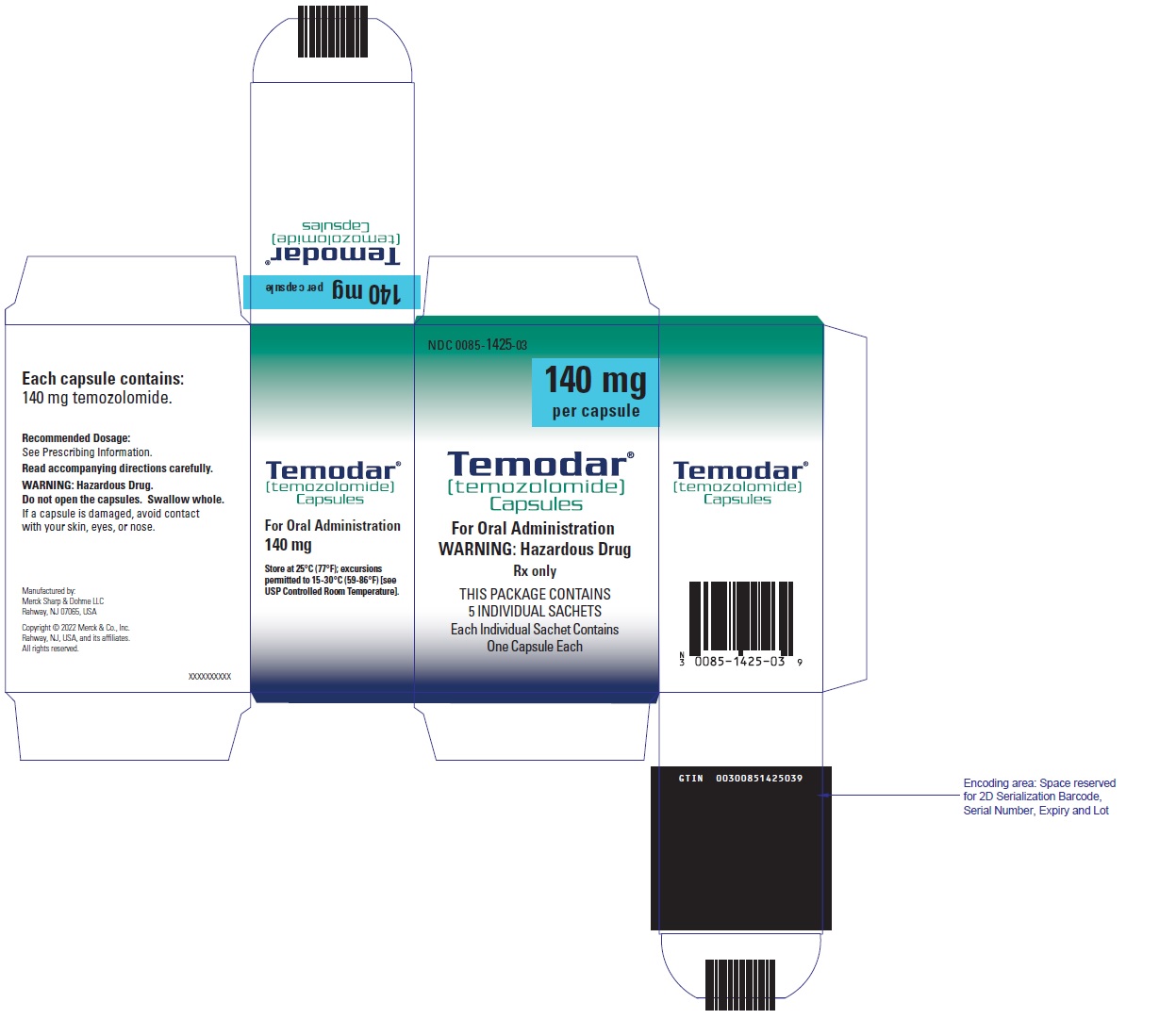
[temozolomide]
Capsules
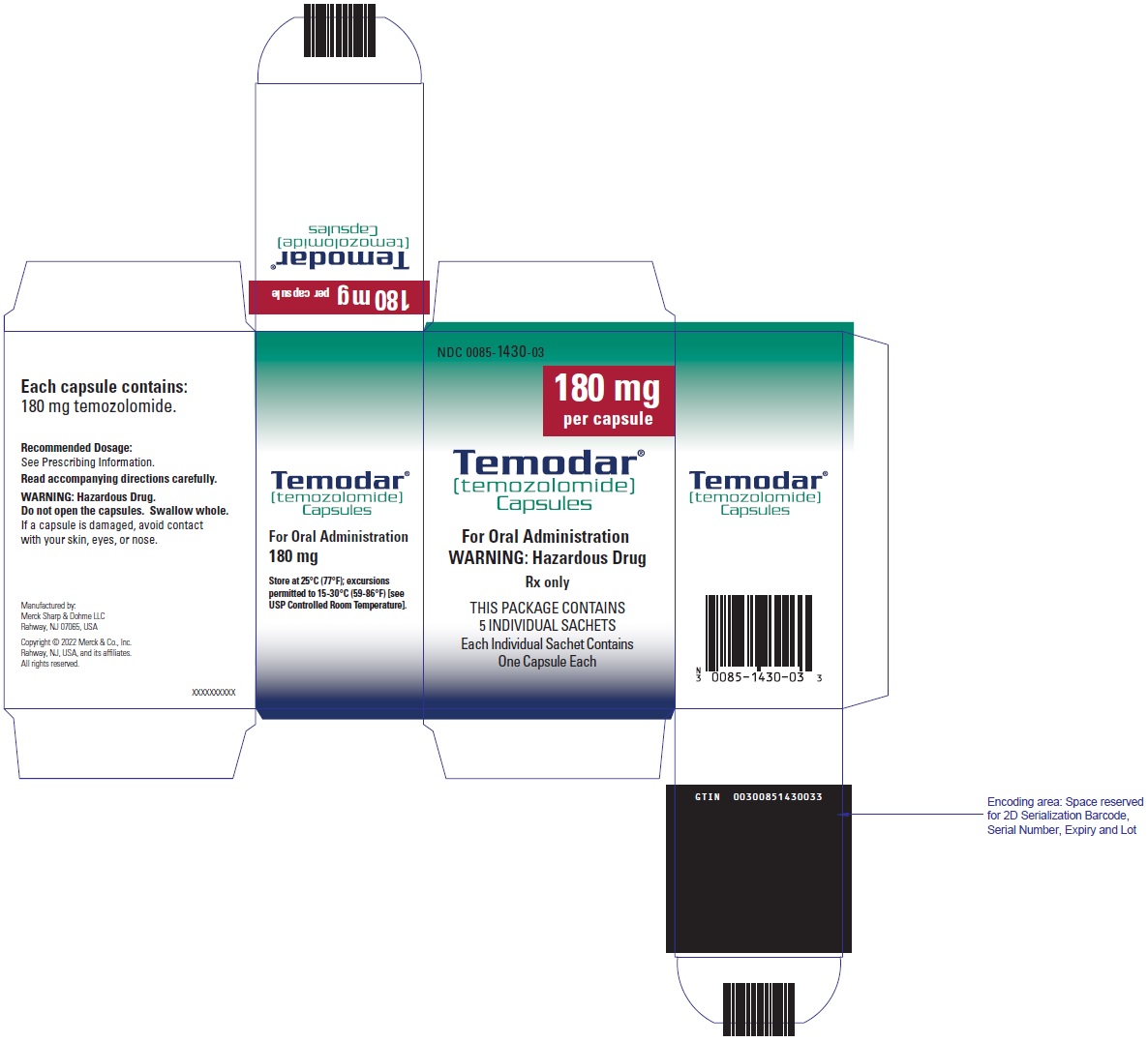
[temozolomide]
Capsules
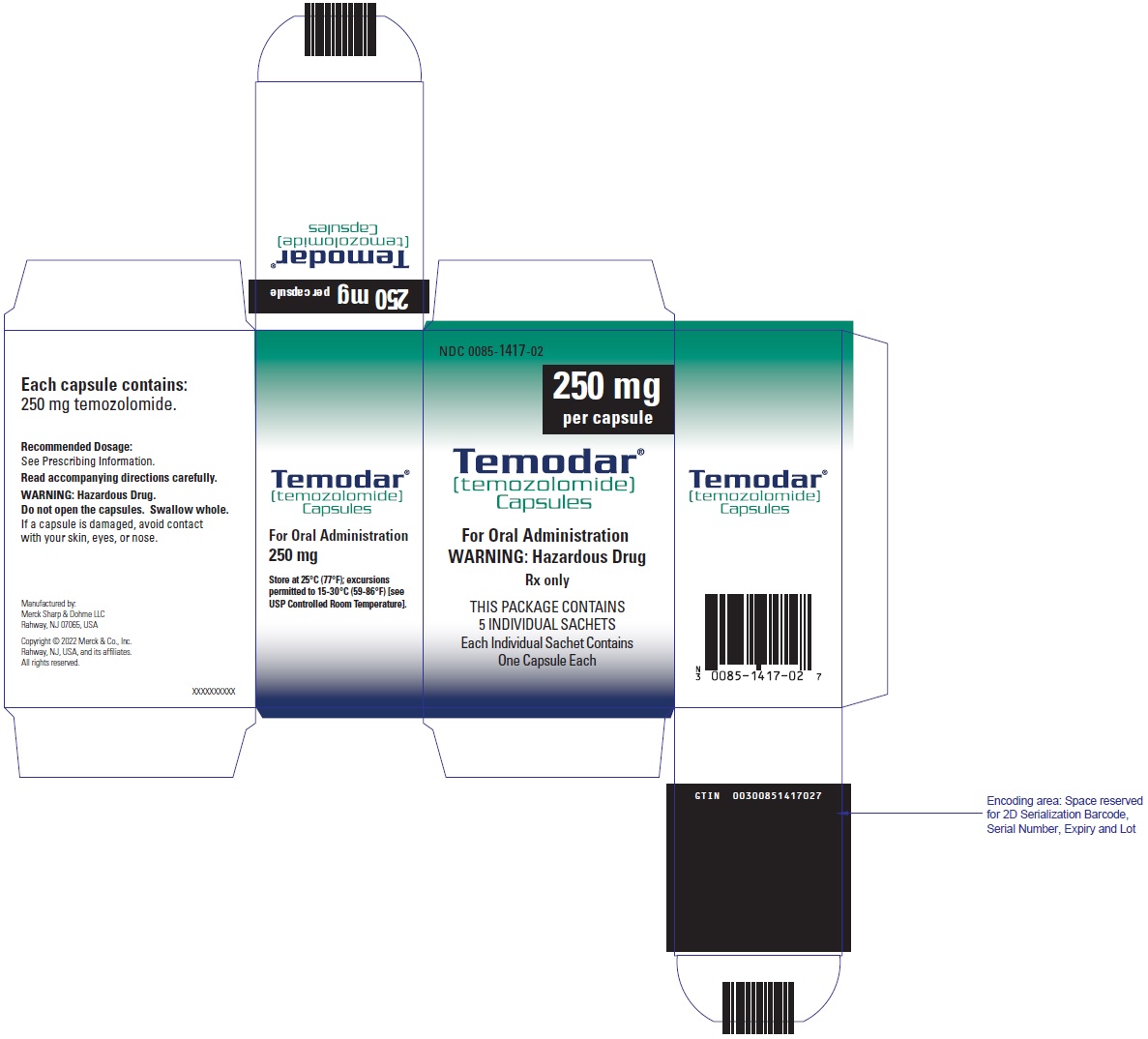
[temozolomide]
for Injection