Tegsedi
What is Tegsedi (Inotersen)?
Living with hereditary transthyretin amyloidosis (hATTR) can be overwhelming. This rare, inherited disorder slowly damages nerves and organs, leading to weakness, pain, numbness, and difficulty with daily activities. For many patients, even simple movements can become a challenge. Tegsedi (inotersen) was developed to help slow this progressive disease, offering patients renewed hope for maintaining independence and quality of life.
Tegsedi is a targeted RNA-based therapy designed to treat polyneuropathy caused by hereditary transthyretin-mediated amyloidosis (hATTR) in adults. It belongs to a class of medications known as antisense oligonucleotides, which work at the genetic level to reduce the production of harmful proteins that cause disease. Approved by the U.S. Food and Drug Administration (FDA) in 2018, Tegsedi represents a specialized and innovative treatment option for people with this rare and often debilitating condition.
What does Tegsedi do?
Tegsedi is used to treat hATTR amyloidosis with polyneuropathy, a genetic disorder in which the liver produces a faulty protein called transthyretin (TTR). This abnormal protein forms sticky deposits known as amyloid, which build up in the body’s tissues and nerves. Over time, these deposits cause nerve damage, leading to symptoms such as pain, numbness, muscle weakness, loss of balance, and problems with digestion or blood pressure regulation.
Tegsedi helps by reducing the amount of TTR protein produced in the liver. Lowering these protein levels slows the formation of amyloid deposits, which in turn helps prevent further nerve damage and may improve nerve function.
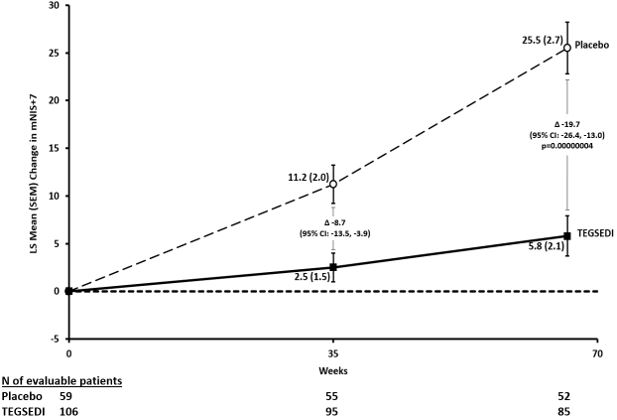
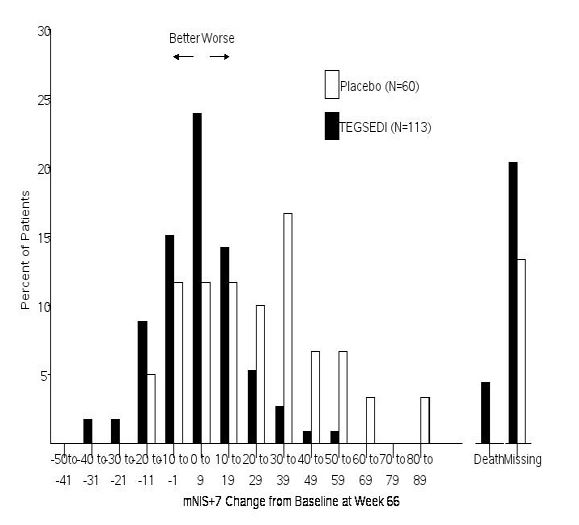
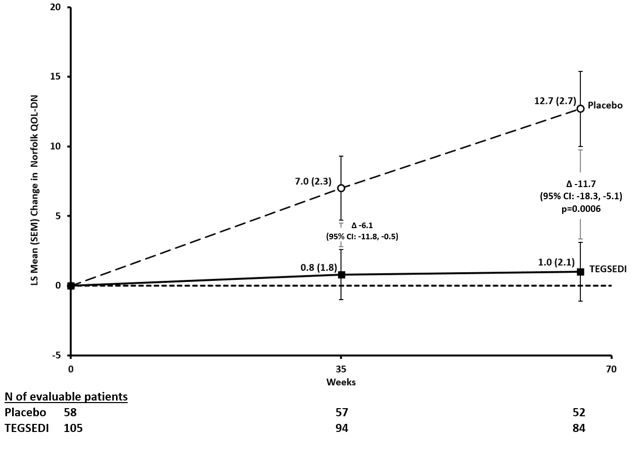
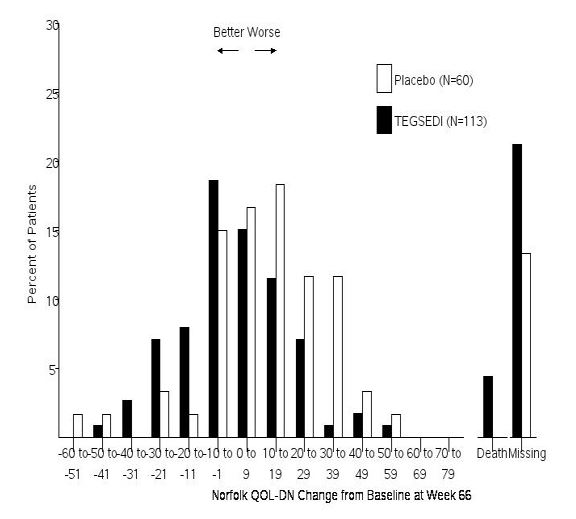
In clinical studies, patients taking Tegsedi showed significant slowing of disease progression compared with those not receiving treatment. Many reported improved ability to walk, less pain, and better control over daily activities. These results demonstrate that, while Tegsedi may not cure hATTR, it can substantially improve both symptoms and quality of life for many patients (FDA, 2024; NIH, 2024).
How does Tegsedi work?
Tegsedi’s active ingredient, inotersen, is an antisense oligonucleotide, a short synthetic strand of genetic material designed to target messenger RNA (mRNA). In simple terms, it works by interfering with the liver’s ability to produce the abnormal transthyretin protein responsible for amyloid buildup.
Here’s how it works step by step:
- Tegsedi binds to the mRNA that carries instructions for making TTR protein.
- Once bound, it signals the body to break down that mRNA, preventing the production of new TTR protein.
- As a result, TTR levels in the blood drop, reducing the amount of amyloid that can form and deposit in tissues.
This mechanism matters clinically because it directly targets the root cause of hATTR, not just the symptoms. By slowing or halting further nerve damage, Tegsedi helps preserve nerve function and mobility.
This precision-based approach represents a major advance in the treatment of genetic amyloidosis and reflects a growing shift toward genomic medicine, where treatments address disease at the molecular level.
Tegsedi side effects
Like all powerful medications, Tegsedi can cause side effects, some of which require careful monitoring. The most important potential risks involve low platelet counts and kidney problems, both of which can be serious if not detected early.
Common side effects include:
- Injection site reactions (pain, redness, or swelling)
- Nausea or vomiting
- Fatigue or headache
- Cough or joint pain
Serious side effects (less common) may include:
- Thrombocytopenia (low platelet count), which may increase the risk of bleeding or bruising
- Glomerulonephritis (kidney inflammation), which can affect kidney function
- Allergic reactions, such as rash, swelling, or difficulty breathing
Tegsedi patients are enrolled in a REMS program due to risks. This requires regular monitoring of platelet counts, kidney function, and liver health through blood and urine tests. Report unusual bruising, nosebleeds, dark urine, swelling, or severe fatigue to your doctor immediately, as these could indicate low platelets or kidney problems.
Patients with preexisting kidney disease or bleeding disorders may not be good candidates for Tegsedi and should discuss alternatives with their healthcare provider.
Tegsedi dosage
Tegsedi is a once-weekly subcutaneous injection, available in pre-filled syringes. Patients or caregivers can be trained for at-home administration, rotating injection sites (abdomen, upper arm, or thigh) to prevent skin irritation.
Regular blood and urine tests are crucial for Tegsedi treatment, monitoring platelet counts, kidney function, and liver enzymes to ensure safety and efficacy. Abnormalities may lead to treatment adjustments or temporary cessation.
Older adults tolerate Tegsedi well but need closer monitoring if they have kidney or blood conditions. Patients must not skip monitoring appointments or self-adjust doses due to potential serious complications.
Does Tegsedi have a generic version?
As of 2025, Tegsedi (inotersen) does not have a generic version available in the United States or internationally. It is marketed exclusively under its brand name by Ionis Pharmaceuticals. However, international versions may exist in other markets.
Generic Tegsedi, if developed, must meet FDA standards for safety, effectiveness, and quality due to its complex RNA-based nature. Patients needing financial aid for Tegsedi can check manufacturer programs or insurance, often with help from providers or pharmacies.
Conclusion
Tegsedi (inotersen) is a breakthrough treatment for adults with hereditary transthyretin-mediated amyloidosis (hATTR) with polyneuropathy. By targeting the disease at its genetic source, it helps reduce the buildup of harmful amyloid deposits and preserve nerve function offering meaningful improvements in mobility, independence, and overall quality of life.
While requiring careful monitoring, Tegsedi effectively slows disease progression and offers hope for patients with this rare condition. When supervised by an experienced provider, it’s a powerful, science-driven treatment. Openly discuss goals, monitoring, and concerns with your medical team. Staying informed, consistent with therapy, and proactive monitoring ensure the safest, most successful outcomes.
References
- U.S. Food and Drug Administration (FDA). (2024). Tegsedi (inotersen) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Inotersen (subcutaneous route) description and precautions. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Inotersen injection: Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Transthyretin amyloidosis and RNA-targeted therapies. Retrieved from https://www.nih.gov
Approved To Treat
Related Clinical Trials
Summary: This is a prospective, non-interventional, Long-term, multinational cohort safety study of patients with Hereditary Transthyretin Amyloidosis with Polyneuropathy (hATTR-PN). The overarching goal of this study is to further characterize the long-term safety of TEGSEDI (inotersen) in patients with hATTR-PN under real-world conditions.
Related Latest Advances
Brand Information
- Platelet count below 100 x 10
- History of acute glomerulonephritis caused by TEGSEDI
- History of a hypersensitivity reaction to TEGSEDI
- Thrombocytopenia
- Glomerulonephritis and Renal Toxicity
- Stroke and Cervicocephalic Arterial Dissection
- Inflammatory and Immune Effects
- Liver Injury
- Hypersensitivity
- Reducted Serum Vitamin A Levels and Recommended Supplementation
- Pack of 1 prefilled syringe: NDC 72126-007-03
- Pack of 4 prefilled syringes: NDC 72126-007-01
- Patients must enroll in the program and comply with ongoing monitoring requirements.
- TEGSEDI is available only from certified pharmacies participating in the program. Therefore, provide patients with the telephone number and website for information on how to obtain the product.







