Reblozyl
What is Reblozyl (Luspatercept)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is a single arm open-label Phase II trial of luspatercept and darbepoetin alfa in non-mutated SF3B1 , lower-risk, RBC transfusion dependent MDS participants with an endogenous erythropoietin (EPO) level \< 500 IU/L.
Summary: The purpose of this Phase 2 study is to evaluate the efficacy and safety of momelotinib (MMB) in combination with luspatercept (LUSPA) in participants with transfusion dependence (TD) primary myelofibrosis (PMF) or Post-polycythemia vera (PV)/ essential thrombocythemia (ET) myelofibrosis (MF) who are either janus kinase (JAK) inhibitor (JAKi) naïve or experienced.
Summary: The purpose of the study is to see if participants with anemia due to their type of MDS or MDS/MPN will experience a more decreased need for regular blood transfusions if they take luspatercept plus best supportive care, and what effect, good and/or bad, luspatercept has on them and their anemia due to MDS or MDS/MPN. The safety and tolerability of luspatercept will also be evaluated in this study...
Related Latest Advances
Brand Information
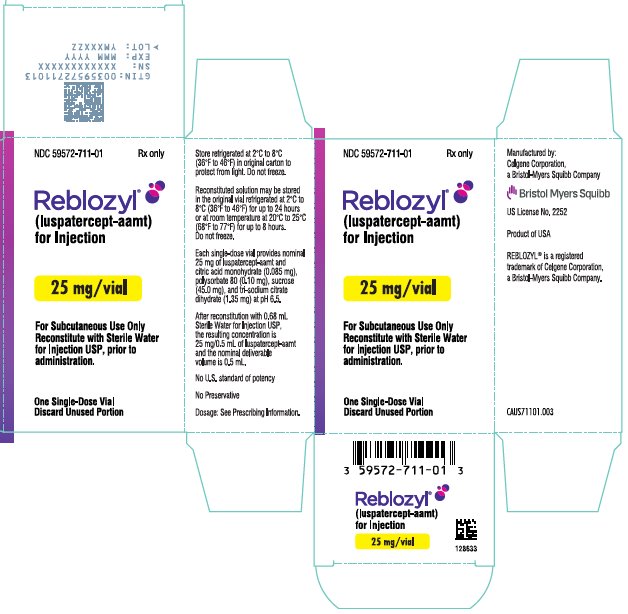
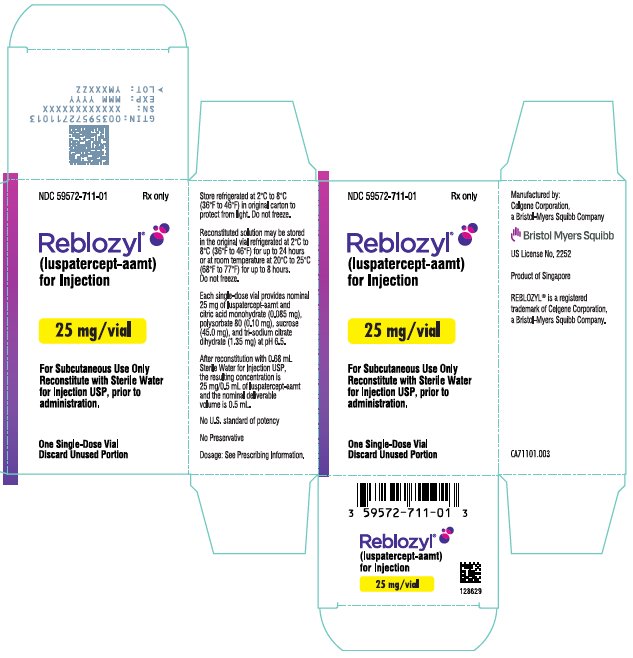
- For injection: 25 mg white to off-white lyophilized powder in a single-dose vial for reconstitution.
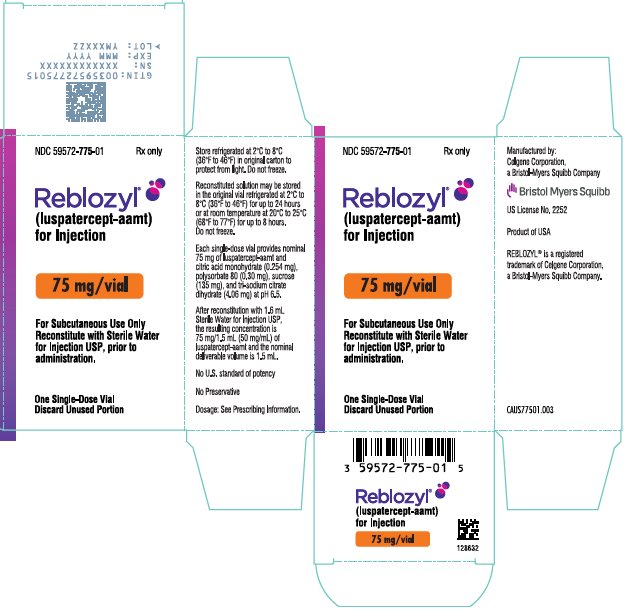
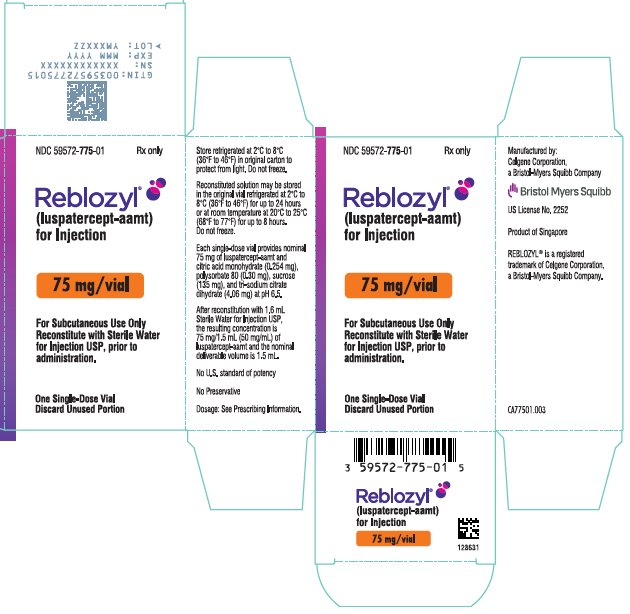
- For injection: 75 mg white to off-white lyophilized powder in a single-dose vial for reconstitution.
- Thrombosis/Thromboembolism
- Hypertension
- Extramedullary Hematopoietic Masses