Brand Name
Firdapse
Generic Name
Amifampridine
View Brand Information FDA approval date: January 07, 2019
Classification: Potassium Channel Blocker
Form: Tablet
What is Firdapse (Amifampridine)?
FIRDAPSE ® is indicated for the treatment of Lambert-Eaton myasthenic syndrome in adults and pediatric patients 6 years of age and older. FIRDAPSE is a potassium channel blocker indicated for the treatment of Lambert-Eaton myasthenic syndrome in adults and pediatric patients 6 years of age and older.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Firdapse (amifampridine phosphate)
1INDICATIONS AND USAGE
FIRDAPSE
2DOSAGE FORMS AND STRENGTHS
FIRDAPSE tablets contain 10 mg amifampridine and are white to off-white, round, and functionally scored. Each tablet is debossed on the non-scored side with “CATALYST” and on the scored side with “211” above the score and “10” below the score.
3CONTRAINDICATIONS
FIRDAPSE is contraindicated in patients with:
- A history of seizures
- Hypersensitivity to amifampridine phosphate or another aminopyridine
4ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Seizures
- Hypersensitivity
5OVERDOSAGE
Overdose with FIRDAPSE was not reported during clinical studies.
In a case report, a 65-year-old patient with LEMS inadvertently received a total daily amifampridine dose of 360 mg/day (approximately 4 times the maximum recommended total daily dose) and was hospitalized for general weakness, paresthesia, nausea, vomiting, and palpitations. The patient developed convulsions and paroxysmal supraventricular tachycardia, and four days after admission, experienced cardiac arrest. The patient was resuscitated and ultimately recovered following withdrawal of amifampridine.
Patients with suspected overdose with FIRDAPSE should be monitored for signs or symptoms of exaggerated FIRDAPSE adverse reactions or effects, and appropriate symptomatic treatment instituted immediately.
6DESCRIPTION
The active ingredient of FIRDAPSE is amifampridine phosphate, which is a voltage-gated potassium channel blocker. Amifampridine phosphate is described chemically as 3,4-diaminopyridine phosphate with a molecular weight of 207.1 and a molecular formula of C
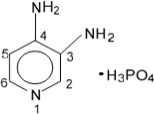
Amifampridine phosphate is a white, crystalline powder that is freely soluble in water, and slightly soluble in solvents ethanol, methanol and acetic acid. A 1% aqueous solution of amifampridine phosphate has a pH of 4.4 at ambient conditions.
Each FIRDAPSE tablet contains 10 mg amifampridine (equivalent to 18.98 mg amifampridine phosphate). The tablet formulation includes the following inactive ingredients: calcium stearate, colloidal silicon dioxide, and microcrystalline cellulose.
FIRDAPSE tablets are intended for oral administration only.
7CLINICAL STUDIES
The efficacy of FIRDAPSE for the treatment of LEMS was demonstrated in two randomized, double-blind, placebo-controlled discontinuation studies. A total of 64 adults (age 21 to 88 years) with LEMS were enrolled (Study 1 and Study 2). The studies enrolled patients with a confirmed diagnosis of LEMS based on either neurophysiology studies or a positive anti-P/Q type voltage-gated calcium channel antibody test. Patients were required to be on an adequate and stable dosage (30 to 80 mg daily) of amifampridine phosphate prior to entering the randomized discontinuation phases of both studies.
The two co-primary measures of efficacy in both studies were the change from baseline to the end of the discontinuation period in the Quantitative Myasthenia Gravis (QMG) score and in the Subject Global Impression (SGI) score.
The QMG is a 13-item physician-rated categorical scale assessing muscle weakness. Each item is assessed on a 4-point scale, where a score of 0 represents no weakness, and a score of 3 represents severe weakness (total score 0-39). Higher scores represent greater impairment.
The SGI is a 7-point scale on which patients rated their global impression of the effects of the study treatment on their physical well- being. Lower scores on the SGI represent lower perceived benefit with the study treatment.
A key secondary efficacy endpoint was the clinical global impression improvement (CGI-I) score, a 7-point scale on which the treating physician rated the global impression of change in clinical symptoms. A higher CGI-I score indicates a perceived worsening of clinical symptoms.
8PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (
9PRINCIPAL DISPLAY PANEL - 10 mg Tablet Blister Pack Carton
Rx Only
120 Tablets Total
FIRDAPSE
Manufactured for:



