Brand Name
Letairis
Generic Name
Ambrisentan
View Brand Information FDA approval date: June 15, 2007
Classification: Endothelin Receptor Antagonist
Form: Tablet
What is Letairis (Ambrisentan)?
Ambrisentan tablets are indicated for the treatment of pulmonary arterial hypertension : To improve exercise ability and delay clinical worsening. Studies establishing effectiveness included predominantly patients with WHO Functional Class II–III symptoms and etiologies of idiopathic or heritable PAH or PAH associated with connective tissue diseases . Ambrisentan tablets are an endothelin receptor antagonist indicated for the treatment of pulmonary arterial hypertension : To improve exercise ability and delay clinical worsening. Studies establishing effectiveness included trials predominantly in patients with WHO Functional Class II–III symptoms and etiologies of idiopathic or heritable PAH or PAH associated with connective tissue diseases .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Letairis (AMBRISENTAN)
WARNING: EMBRYO-FETAL TOXICITY
Letairis is contraindicated for use during pregnancy because it may cause major birth defects if used by pregnant patients, based on studies in animals
Therefore, for females of reproductive potential, exclude pregnancy before the initiation of treatment with Letairis. Advise use of effective contraception before initiation, during treatment, and for one month after treatment with Letairis
1INDICATIONS AND USAGE
Letairis is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) in adult patients:
- To improve exercise ability and delay clinical worsening.
- In combination with tadalafil to reduce the risks of disease progression and hospitalization for worsening PAH, and to improve exercise ability
Studies establishing effectiveness included predominantly patients with WHO Functional Class II–III symptoms and etiologies of idiopathic or heritable PAH (60%) or PAH associated with connective tissue diseases (34%).
2DOSAGE FORMS AND STRENGTHS
5 mg and 10 mg film
- Each 5 mg tablet is square convex, pale pink, with “5” on one side and “GSI” on the other side.
- Each 10 mg tablet is oval convex, deep pink, with “10” on one side and “GSI” on the other side.
3ADVERSE REACTIONS
Clinically significant adverse reactions that appear in other sections of the labeling include:
- Embryo-fetal Toxicity
- Fluid Retention
- Pulmonary Edema with PVOD
- Decreased Sperm Count
- Hematologic Changes
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Safety data for Letairis are presented from two 12-week, placebo-controlled studies (ARIES-1 and ARIES-2) in patients with pulmonary arterial hypertension (PAH), and one randomized, double-blind, active-controlled trial in 605 patients with PAH (AMBITION) comparing Letairis plus tadalafil to Letairis or tadalafil alone. The exposure to Letairis in these studies ranged from 1 day to 4 years (N=357 for at least 6 months and N=279 for at least 1 year).
In ARIES-1 and ARIES-2, a total of 261 patients received Letairis at doses of 2.5, 5, or 10 mg once daily and 132 patients received placebo. The adverse reactions that occurred in >3% more patients receiving Letairis than receiving placebo are shown in Table 1.
Most adverse drug reactions were mild to moderate and only nasal congestion was dose-dependent.
Few notable differences in the incidence of adverse reactions were observed for patients by age or sex. Peripheral edema was similar in younger patients (<65 years) receiving Letairis (14%; 29/205) or placebo (13%; 13/104), and was greater in elderly patients (≥65 years) receiving Letairis (29%; 16/56) compared to placebo (4%; 1/28). The results of such subgroup analyses must be interpreted cautiously.
The incidence of treatment discontinuations due to adverse events other than those related to PAH during the clinical trials in patients with PAH was similar for Letairis (2%; 5/261 patients) and placebo (2%; 3/132 patients). The incidence of patients with serious adverse events other than those related to PAH during the clinical trials in patients with PAH was similar for placebo (7%; 9/132 patients) and for Letairis (5%; 13/261 patients).
During 12-week controlled clinical trials, the incidence of aminotransferase elevations >3 × upper limit of normal (ULN) were 0% on Letairis and 2.3% on placebo. In practice, cases of hepatic injury should be carefully evaluated for cause.
3.2Postmarketing Experience
The following adverse reactions were identified during post-approval use of Letairis. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to estimate reliably the frequency or to establish a causal relationship to drug exposure: anemia requiring transfusion
Elevations of liver aminotransferases (ALT, AST) have been reported with Letairis use; in most cases alternative causes of the liver injury could be identified (heart failure, hepatic congestion, hepatitis, alcohol use, hepatotoxic medications). Other endothelin receptor antagonists have been associated with elevations of aminotransferases, hepatotoxicity, and cases of liver failure
4DRUG INTERACTIONS
Multiple dose coadministration of ambrisentan and cyclosporine resulted in an approximately 2-fold increase in ambrisentan exposure in healthy volunteers; therefore, limit the dose of ambrisentan to 5 mg once daily when coadministered with cyclosporine
5OVERDOSAGE
There is no experience with overdosage of Letairis. The highest single dose of Letairis administered to healthy volunteers was 100 mg, and the highest daily dose administered to patients with PAH was 10 mg once daily. In healthy volunteers, single doses of 50 mg and 100 mg (5 to 10 times the maximum recommended dose) were associated with headache, flushing, dizziness, nausea, and nasal congestion. Massive overdosage could potentially result in hypotension that may require intervention.
6DESCRIPTION
Letairis contains ambrisentan, an endothelin receptor antagonist. The chemical name ofambrisentan is (+)-(2
Figure 1 Ambrisentan Structural Formula
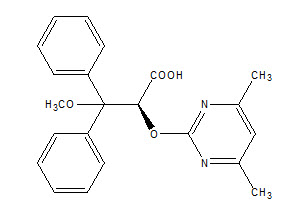
Ambrisentan is a white to off-white, crystalline solid. It is a carboxylic acid with a pKa of 4.0. Ambrisentan is practically insoluble in water and in aqueous solutions at low pH. Solubility increases in aqueous solutions at higher pH. In the solid state ambrisentan is very stable, is not hygroscopic, and is not light sensitive.
Letairis is available as 5 mg and 10 mg film-coated tablets for once daily oral administration. The tablets include the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate and microcrystalline cellulose. The tablets are film-coated with a coating material containing FD&C Red #40 aluminum lake, lecithin, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. Each square, pale pink Letairis tablet contains 5 mg of ambrisentan. Each oval, deep pink Letairis tablet contains 10 mg of ambrisentan. Letairis tablets are unscored.
7HOW SUPPLIED/STORAGE AND HANDLING
Letairis film
8PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (
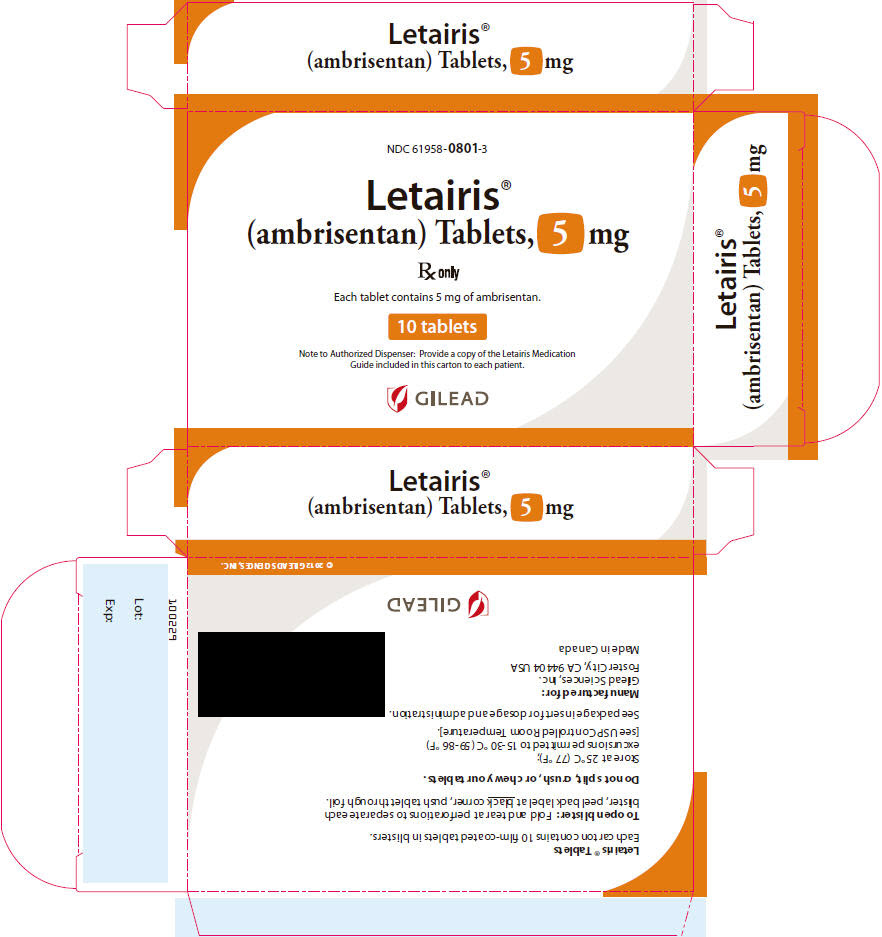
9PRINCIPAL DISPLAY PANEL - 5 mg Tablet Carton
NDC 61958-
Letairis
R
Each tablet contains 5 mg of ambrisentan.
10 tablets
Note to Authorized Dispenser: Provide a copy of the Letairis Medication
GILEAD
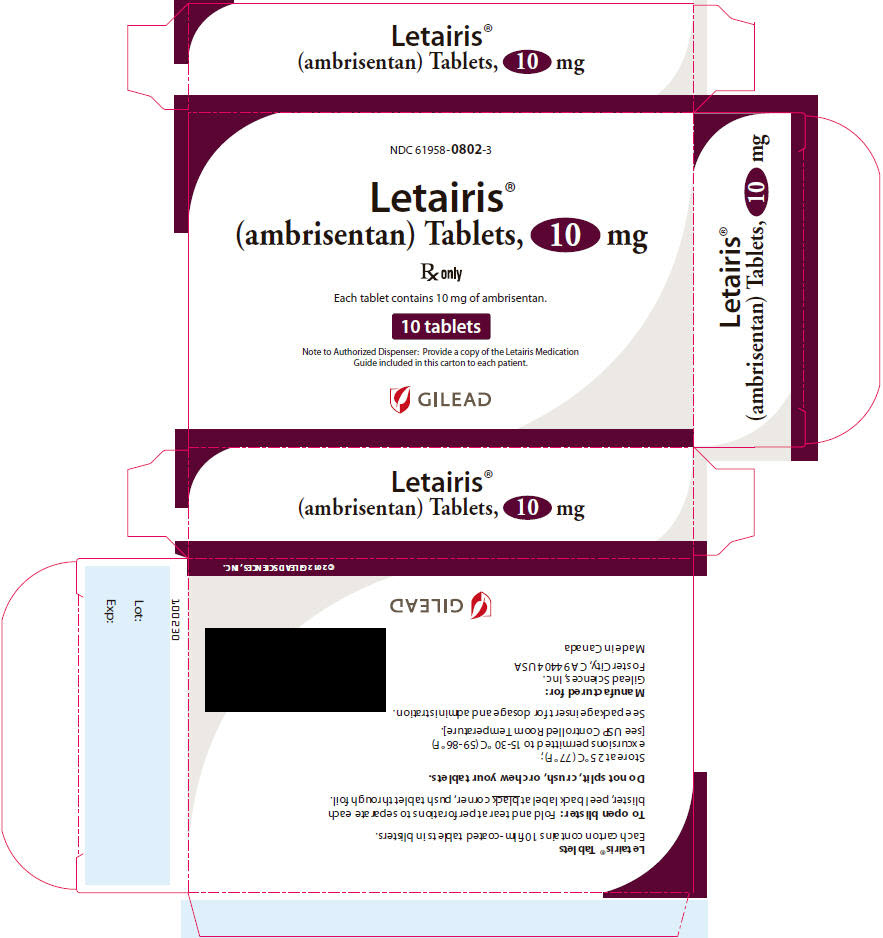
10PRINCIPAL DISPLAY PANEL - 10 mg Tablet Carton
NDC 61958-
Letairis
R
Each tablet contains 10 mg of ambrisentan.
10 tablets
Note to Authorized Dispenser: Provide a copy of the Letairis Medication
GILEAD