Ondansetron
What is Ondansetron ODT (Ondansetron)?
Nausea and vomiting can make recovery from illness or treatment incredibly difficult. Whether it’s caused by chemotherapy, surgery, or severe gastroenteritis, these symptoms can drain strength, delay healing, and reduce quality of life. Ondansetron ODT (orally disintegrating tablet) helps bring relief to patients facing these challenges by preventing nausea and vomiting before they start.
Ondansetron ODT belongs to a class of medications called serotonin 5-HT₃ receptor antagonists, or simply antiemetics. It works by blocking certain chemical signals in the body that trigger nausea and vomiting. The ODT (orally disintegrating tablet) form dissolves quickly on the tongue without the need for water, making it especially useful for patients who can’t swallow pills or are actively feeling sick.
First approved by the U.S. Food and Drug Administration (FDA) in the early 1990s, ondansetron has become a trusted, first-line medication for preventing nausea and vomiting in both adults and children undergoing chemotherapy, radiation therapy, or surgery. Its safety, effectiveness, and convenience make it a mainstay in modern supportive care.
What does Ondansetron ODT do?
Ondansetron ODT is prescribed to prevent and treat nausea and vomiting caused by various medical treatments and conditions. Its most common uses include:
- Chemotherapy-induced nausea and vomiting (CINV): Many cancer patients receive ondansetron before chemotherapy to prevent the intense nausea that certain chemotherapy drugs can trigger.
- Postoperative nausea and vomiting (PONV): After surgery, some people experience nausea due to anesthesia or pain medications. Ondansetron helps reduce this risk.
- Radiation therapy–related nausea: It can help patients undergoing radiation treatment, especially those receiving therapy near the stomach or brain.
- Gastroenteritis or viral infections: In some cases, doctors may prescribe ondansetron to help manage severe vomiting so that patients can stay hydrated.
Clinical studies have shown that ondansetron significantly reduces both the frequency and severity of nausea and vomiting compared with placebo (NIH, 2024). Patients typically report relief within 30 minutes of taking the medication, allowing them to eat, drink, and recover more comfortably.
How does Ondansetron ODT work?
Ondansetron ODT works by blocking serotonin (5-HT₃), a natural chemical messenger involved in triggering nausea and vomiting.
During chemotherapy, surgery, or gastrointestinal irritation, the body releases serotonin in the gut. This serotonin activates 5-HT₃ receptors in the brain’s vomiting center and along the vagus nerve, sending signals that cause nausea and the urge to vomit. Ondansetron blocks these receptors, preventing those signals from reaching the brain.
By interrupting this pathway, ondansetron helps patients feel more stable and comfortable during treatment or recovery. Clinically, this is important because preventing nausea not only improves comfort but also helps patients stick to their prescribed treatments, especially in cancer therapy where maintaining nutrition and hydration is critical.
The ODT form offers added convenience, it dissolves on the tongue within seconds, making it easy for patients who can’t keep water down or have difficulty swallowing pills.
Ondansetron ODT side effects
Most people tolerate ondansetron ODT well, but like all medications, it can cause side effects.
Common side effects include:
- Headache
- Fatigue or mild drowsiness
- Constipation
- Dizziness
- Flushing or a warm feeling
Less common but potentially serious side effects:
- Irregular heartbeat or chest pain
- Fainting or severe dizziness
- Blurred vision or temporary vision loss (rare, usually with IV use)
- Signs of an allergic reaction such as rash, itching, or difficulty breathing
Ondansetron can affect heart rhythm, so those with congenital long QT syndrome, heart disease, or on QT-prolonging medications need medical supervision. Patients with liver impairment may require lower doses. Those with severe constipation or intestinal blockage should discuss risks before treatment.
If severe dizziness, fainting, or signs of allergic reaction occur, medical attention should be sought immediately. Fortunately, such reactions are rare, and ondansetron is considered a safe and well-tolerated medication when used as directed.
Ondansetron ODT dosage
Ondansetron ODT is a dissolvable tablet taken by mouth, once or multiple times daily, depending on the cause of nausea. Strengths vary based on patient needs. Let the tablet dissolve completely, peel back foil to remove, and store in a cool, dry place, handling with dry hands.
Doctors may monitor electrolytes and heart rhythm in patients with cardiac history or on multiple medications. Liver disease patients may require dosage adjustments. Ondansetron is safe for older adults and children, with healthcare provider-guided dosing.
Does Ondansetron ODT have a generic version?
Yes. Ondansetron ODT is available in generic form and is FDA-approved in the United States. The generic version contains the same active ingredient, strength, and effectiveness as the brand-name drug, Zofran ODT.
Generic ondansetron ODT is a more affordable, equally safe, and effective alternative to the brand-name version, meeting FDA standards. Other forms like oral tablets, solutions, and injectables are also available for different patient needs.
Conclusion
Ondansetron ODT is a trusted and effective medication that provides fast relief from nausea and vomiting caused by chemotherapy, surgery, or other medical conditions. By blocking serotonin receptors that trigger these symptoms, it helps patients maintain comfort, hydration, and energy during recovery or ongoing treatment.
Ondansetron ODT, an easy-to-take dissolving tablet, helps those unable to swallow pills or keep liquids down. Side effects are usually mild and temporary, and communication with a healthcare provider ensures safe and effective treatment. Used in various settings, it offers relief, allowing patients to focus on healing..
References
- U.S. Food and Drug Administration (FDA). (2024). Ondansetron ODT prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Ondansetron (oral route) description and precautions. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Ondansetron: Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Serotonin receptor antagonists and antiemetic therapy. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
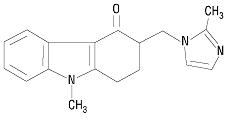
The molecular formula is C18H19N3O representing a molecular weight of 293.4 g/mol. Ondansetron is a white to off-white powder.
Each 4 mg ondansetron orally disintegrating tablet, USP for oral administration contains 4 mg ondansetron base. Each 8 mg ondansetron orally disintegrating tablet, USP for oral administration contains 8 mg ondansetron base. Each ondansetron orally disintegrating tablet, USP also contains the inactive ingredients mannitol, crospovidone, lactose monohydrate, microcrystalline cellulose, aspartame, strawberry guarana flavor, colloidal silicon dioxide, and magnesium stearate. The strawberry guarana flavor contains maltodextrin, propylene glycol, artificial flavors, and acetic acid. Ondansetron orally disintegrating tablets, USP are orally administered formulation of ondansetron which disintegrates on the tongue and does not require water to aid dissolution or swallowing.
Meets USP Disintegration Test 2.
No trials have been performed in males.

