Brand Name
Darifenacin
View Brand InformationFDA approval date: September 01, 2016
Classification: Cholinergic Muscarinic Antagonist
Form: Tablet
What is Darifenacin?
Darifenacin extended-release tablets are muscarinic antagonist indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency and frequency. Darifenacin extended-release tablets are indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency and frequency.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Randomized, Double-blind, Single Center, Phase 2 Study to Assess the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of 15 mg of Darifenacin Daily in Patients With Amyotrophic Lateral Sclerosis
Summary: Amyotrophic lateral sclerosis (ALS) is a progressive neurological disorder characterized by selective death of upper and lower motor neurons, which leads to severe disability and fatal outcomes. One of the major hallmarks of ALS is the denervation of neuromuscular junctions (NMJs), which is one of the earliest events seen in ALS patients and mouse models of ALS. Under healthy conditions, glial cel...
Related Latest Advances
Brand Information
Darifenacin (Darifenacin)
1INDICATIONS AND USAGE
Darifenacin extended-release tablets are indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency and frequency.
2DOSAGE AND ADMINISTRATION
The recommended starting dose of darifenacin extended-release tablets is 7.5 mg orally once daily. Based upon individual response, the dose may be increased to 15 mg once daily, as early as two weeks after starting therapy.
3DOSAGE FORMS AND STRENGTHS
Darifenacin extended-release tablets 7.5 mg are white colored, round convex, film-coated tablets debossed with “Z” on one side and “22” on other side.
4CONTRAINDICATIONS
Darifenacin extended-release tablets are contraindicated in patients with, or at risk for, the following conditions:
- urinary retention
- gastric retention, or
- uncontrolled narrow-angle glaucoma.
5OVERDOSAGE
Overdosage with antimuscarinic agents, including darifenacin extended-release tablets, can result in severe antimuscarinic effects. Treatment should be symptomatic and supportive. In the event of overdosage, ECG monitoring is recommended. Darifenacin extended-release tablets have been administered in clinical trials at doses up to 75 mg (five times the maximum therapeutic dose) and signs of overdose were limited to abnormal vision.
6DESCRIPTION
Darifenacin is an extended-release tablet for oral administration which contains 7.5 mg or 15 mg darifenacin as its hydrobromide salt. The active moiety, darifenacin, is a potent muscarinic receptor antagonist.
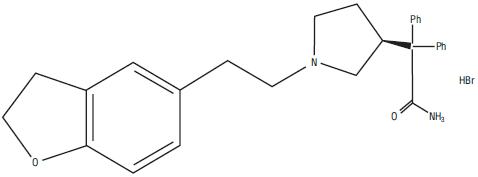
Darifenacin hydrobromide is a white to off -white powder, with a molecular weight of 507.5.
7CLINICAL STUDIES
Darifenacin extended-release tablets were evaluated for the treatment of patients with overactive bladder with symptoms of urgency, urge urinary incontinence, and increased urinary frequency in three randomized, fixed-dose, placebo-controlled, multicenter, double-blind, 12-week studies (Studies 1, 2 and 3) and one randomized, double-blind, placebo-controlled, multicenter, dose-titration study (Study 4). For study eligibility in all four studies, patients with symptoms of overactive bladder for at least six months were required to demonstrate at least eight micturitions and at least one episode of urinary urgency per day, and at least five episodes of urge urinary incontinence per week. The majority of patients were white (94%) and female (84%), with a mean age of 58 years, range 19 to 93 years. Thirty-three percent of patients were greater than or equal to 65 years of age
Table 4 shows the efficacy data collected from 7- or 14-day voiding diaries in the three fixed-dose placebo-controlled studies of 1,059 patients treated with placebo, 7.5 mg or 15 mg once daily darifenacin extended-release tablets for 12 weeks. A significant decrease in the primary endpoint, change from baseline in average weekly urge urinary incontinence episodes was observed in all three studies. Data is also shown for two secondary endpoints, change from baseline in the average number of micturitions per day (urinary frequency) and change from baseline in the average volume voided per micturition.
Table 5 shows the efficacy data from the dose-titration study in 395 patients who initially received 7.5 mg darifenacin extended-release tablets or placebo daily with the option to increase to 15 mg darifenacin extended-release tablets or placebo daily after two weeks.
As seen in Figures 2 a, 2b and 2c, reductions in the number of urge incontinence episodes per week were observed within the first two weeks in patients treated with darifenacin extended-release tablets 7.5 mg and 15 mg once daily compared to placebo. Further, these effects were sustained throughout the 12-week treatment period.
8HOW SUPPLIED/STORAGE AND HANDLING
Darifenacin Extended-Release Tablets, 7.5 mg are white colored, round convex, film-coated tablets debossed with “Z” on one side and “22” on other side.
Bottles of 30 NDC 65862-861-30
Bottles of 90 NDC 65862-861-90
Bottles of 500 NDC 65862-861-05
Bottles of 1,000 NDC 65862-861-99
Darifenacin Extended-Release Tablets, 15 mg are light peach colored, round convex, film-coated tablets debossed with “Z” on one side and “23” on other side.
Bottles of 30 NDC 65862-862-30
Bottles of 90 NDC 65862-862-90
Bottles of 500 NDC 65862-862-05
Bottles of 1,000 NDC 65862-862-99
Storage
Store at 20° to 25°C (68° to 77° F) [see USP Controlled Room Temperature]. Protect from light.
Keep this and all drugs out of the reach of children.
Bottles of 30 NDC 65862-861-30
Bottles of 90 NDC 65862-861-90
Bottles of 500 NDC 65862-861-05
Bottles of 1,000 NDC 65862-861-99
Darifenacin Extended-Release Tablets, 15 mg are light peach colored, round convex, film-coated tablets debossed with “Z” on one side and “23” on other side.
Bottles of 30 NDC 65862-862-30
Bottles of 90 NDC 65862-862-90
Bottles of 500 NDC 65862-862-05
Bottles of 1,000 NDC 65862-862-99
Storage
Store at 20° to 25°C (68° to 77° F) [see USP Controlled Room Temperature]. Protect from light.
Keep this and all drugs out of the reach of children.
9PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (
Patients should be informed that darifenacin may produce clinically significant angioedema that may result in airway obstruction. Patients should be advised to promptly discontinue darifenacin therapy and seek immediate medical attention if they experience edema of the tongue or laryngopharynx, or difficulty breathing.
Darifenacin extended-release tablets should be taken once daily with water. They may be taken with or without food, and should be swallowed whole and not chewed, divided or crushed.
10FDA-Approved Patient Labeling
Patient InformationDarifenacin Extended-Release Tablets
[Darifenacin (dar e FEN a sin)]
[Darifenacin (dar e FEN a sin)]
Read this Patient Information leaflet about darifenacin extended-release tablets before you start taking them and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your doctor about your medical condition or your treatment.
What are darifenacin extended-release tablets?
Darifenacin extended-release tablets are a prescription medicine for adults used to treat the following symptoms due to a condition called overactive bladder:
What are darifenacin extended-release tablets?
Darifenacin extended-release tablets are a prescription medicine for adults used to treat the following symptoms due to a condition called overactive bladder:
- Urge urinary incontinence: a strong need to urinate with leaking or wetting accidents
- Urgency: a strong need to urinate right away
- Frequency: urinating often
It is unknown if darifenacin extended-release tablets are safe and effective in children.
- are not able to empty your bladder (“urinary retention”)
- have delayed or slow emptying of your stomach (“gastric retention”)
- have an eye problem called “uncontrolled narrow-angle glaucoma”
What should I tell my healthcare provider before starting darifenacin extended-release tablets?
Before starting darifenacin extended-release tablets, tell your doctor if you:
Before starting darifenacin extended-release tablets, tell your doctor if you:
- have trouble emptying your bladder or if you have a weak urine stream
- have any stomach or intestinal problems, or problems with constipation
- have liver problems
- have any other medical conditions
- are pregnant or are planning to become pregnant. It is not known if darifenacin extended-release tablets can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if darifenacin passes into breast milk and if it can harm your baby. Talk to your doctor about the best way to feed your baby if you take darifenacin extended-release tablets.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Darifenacin extended-release tablets and certain other medicines may affect each other, causing side effects.
Especially tell your healthcare provider if you take a:
Especially tell your healthcare provider if you take a:
- antifungal medicine ketoconazole (Nizoral
- antibiotic medicine clarithromycin (Biaxin
- anti-HIV medicine ritonavir (Norvir
- medicine to treat depression nefazadone (Serzone
- medicine to treat an abnormal heartbeat flecainide (Tambocor™)
- antipsychotic medicine thioridazine (Mellaril
- medicine to treat depression called a tricyclic antidepressant
Know all the medicines you take. Keep a list of them with you to show your doctor and pharmacist each time you get a new medicine.
- Take darifenacin extended-release tablets exactly as prescribed. Your doctor will prescribe the dose that is right for you. Take darifenacin extended-release tablets 1 time daily with water.
- Darifenacin extended-release tablets should be swallowed whole. Do not chew, cut or crush darifenacin extended-release tablet.
- Darifenacin extended-release tablets may be taken with or without food.
- If you take too much darifenacin call your doctor or go to the nearest hospital emergency room right away.
What should I avoid while taking darifenacin extended-release tablets?
Darifenacin extended-release tablets can cause blurred vision or dizziness. Do not drive or operate heavy machinery until you know how darifenacin extended-release tablets affect you.
What are the possible side effects of darifenacin extended-release tablets?
Darifenacin extended-release tablets may cause serious side effects including:
Darifenacin extended-release tablets can cause blurred vision or dizziness. Do not drive or operate heavy machinery until you know how darifenacin extended-release tablets affect you.
What are the possible side effects of darifenacin extended-release tablets?
Darifenacin extended-release tablets may cause serious side effects including:
- Serious allergic reaction. Stop taking darifenacin extended-release tablets and get medical help right away if you have:
The most common side effects with darifenacin extended-release tablets are:
- constipation
- dry mouth
- headache
- heartburn
- nausea
- urinary tract infection
- blurred vision
- heat exhaustion or heat-stroke. This can happen when darifenacin extended-release tablets are used in hot environments. Symptoms of heat exhaustion may include:
Tell your doctor if you have any side effect that bothers you or that does not go away.
- Store darifenacin extended-release tablets at room temperature, between 20° to 25°C (68° to 77°F).
- Keep darifenacin extended-release tablets out of the light.
Keep darifenacin extended-release tablets and all medicines out of the reach of children.
General information about darifenacin extended-release tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use darifenacin extended-release tablets for a condition for which it was not prescribed. Do not give darifenacin extended-release tablets to other people, even if they have the same symptoms you have. They may harm them.
This Patient Information leaflet summarizes the most important information about darifenacin extended-release tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about darifenacin extended-release tablets that is written for health professionals.
What are the ingredients in darifenacin extended-release tablets?
Active ingredient: darifenacin hydrobromide
Inactive ingredients: colloidal silicon dioxide, dibasic calcium phosphate dihydrate, ethyl cellulose, hydroxy ethyl cellulose, hydroxy propyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, povidone, talc, and titanium dioxide. In addition, the 15 mg tablet also contains red iron oxide and yellow iron oxide.
The brands listed are the trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
Distributed by:
Aurobindo Pharma USA, Inc.279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited Hyderabad-500 032, India
Revised: 09/2021
General information about darifenacin extended-release tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use darifenacin extended-release tablets for a condition for which it was not prescribed. Do not give darifenacin extended-release tablets to other people, even if they have the same symptoms you have. They may harm them.
This Patient Information leaflet summarizes the most important information about darifenacin extended-release tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about darifenacin extended-release tablets that is written for health professionals.
What are the ingredients in darifenacin extended-release tablets?
Active ingredient: darifenacin hydrobromide
Inactive ingredients: colloidal silicon dioxide, dibasic calcium phosphate dihydrate, ethyl cellulose, hydroxy ethyl cellulose, hydroxy propyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, povidone, talc, and titanium dioxide. In addition, the 15 mg tablet also contains red iron oxide and yellow iron oxide.
The brands listed are the trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited.
Distributed by:
Aurobindo Pharma USA, Inc.279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited Hyderabad-500 032, India
Revised: 09/2021
11PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 7.5 mg (30 Tablet Bottle)
NDC 65862-861-30
Darifenacin Extended-release Tablets7.5 mgAUROBINDO 30 Tablets
Darifenacin Extended-release Tablets7.5 mgAUROBINDO 30 Tablets
12PACKAGE LABEL-PRINCIPAL DISPLAY PANEL- 15 mg (30 Tablet Bottle)
NDC 65862-862-30
Darifenacin Extended-release Tablets15 mgAUROBINDO 30 Tablets
Darifenacin Extended-release Tablets15 mgAUROBINDO 30 Tablets

