Brand Name
Almotriptan
View Brand InformationFDA approval date: July 07, 2015
Classification: Serotonin-1b and Serotonin-1d Receptor Agonist
Form: Tablet
What is Almotriptan?
Almotriptan malate tablets are a 5HT 1B/1D receptor agonist indicated for: Acute treatment of migraine attacks in adults with a history of migraine with or without aura.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Almotriptan Malate (Almotriptan Malate)
1DOSAGE FORMS AND STRENGTHS
Almotriptan malate tablets are available as white, film-coated, round, convex tablets in the following strengths:
6.25 mg tablet debossed with “93” on one side and “A1” on the other side
12.5 mg tablet debossed with “93” on one side and “A2” on the other side
2ADVERSE REACTIONS
Serious cardiac reactions, including myocardial infarction, have occurred following the use of almotriptan malate tablets. These reactions are extremely rare and most have been reported in patients with risk factors predictive of CAD. Reactions reported in association with triptans have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Risk of Myocardial Ischemia and Infarction and Other Adverse Cardiac Events
- Sensations of Pain, Tightness, Pressure in the Chest and/or Throat, Neck, and Jaw
- Cerebrovascular Events and Fatalities
- Other Vasospasm-Related Events, including Peripheral Vascular Ischemia and Colonic Ischemia
- Serotonin Syndrome
- Increases in Blood Pressure
Adverse events were assessed in controlled clinical trials that included 1840 adult patients who received one or two doses of almotriptan malate and 386 adult patients who received placebo. The most common adverse reactions during treatment with almotriptan malate were nausea, somnolence, headache, paresthesia, and dry mouth. In long-term open-label studies where patients were allowed to treat multiple attacks for up to 1 year, 5% (63 out of 1347 patients) withdrew due to adverse experiences.
Adverse events were assessed in controlled clinical trials that included 362 adolescent patients who received almotriptan malate and 172 adolescent patients who received placebo. The most common adverse reactions during treatment with almotriptan malate were dizziness, somnolence, headache, paresthesia, nausea, and vomiting. In a long-term, open-label study where patients were allowed to treat multiple attacks for up to 1 year, 2% (10 out of 420 adolescent patients) withdrew due to adverse events.
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
2.1Commonly-Observed Adverse Reactions in Double-Blind, Placebo-Controlled Almotriptan Malate Clinical Trials
Adults
Table 1 lists the adverse events that occurred in at least 1% of the adult patients treated with almotriptan malate, and at an incidence greater than in patients treated with placebo, regardless of drug relationship.
The incidence of adverse events in controlled clinical trials was not affected by gender, weight, age, presence of aura, or use of prophylactic medications or oral contraceptives. There were insufficient data to assess the effect of race on the incidence of adverse events.
Adolescents
Table 2 lists the adverse reactions reported by 1% or more of almotriptan malate-treated adolescents age 12 to 17 years in 1 placebo-controlled, double-blind clinical trial.
2.2Other Adverse Reactions Observed in Almotriptan Malate Clinical Trials
In the paragraphs that follow, the frequencies of less commonly reported adverse clinical reactions are presented. The reports include adverse reactions in 5 adult controlled studies and 1 adolescent controlled study. Variability associated with adverse reaction reporting, the terminology used to describe adverse reactions, etc., limit the value of the quantitative frequency estimates provided. Reaction frequencies are calculated as the number of patients who used almotriptan malate and reported a reaction divided by the total number of patients exposed to almotriptan malate (n = 3047, all doses). All reported reactions are included except those already listed in the previous table, those too general to be informative, and those not reasonably associated with the use of the drug. Reactions are further classified within system organ class and enumerated in order of decreasing frequency using the following definitions: frequent adverse reactions are those occurring in 1/100 or more patients, infrequent adverse reactions are those occurring in fewer than 1/100 to 1/1000 patients, and rare adverse reactions are those occurring in fewer than 1/1000 patients.
Body:
Cardiovascular:
Digestive:
Metabolic:
Musculo-Skeletal:
Nervous:
Respiratory:
Skin:
Special Senses:
Urogenital:
2.3Postmarketing Experience
The following adverse reactions have been identified during postapproval use of almotriptan malate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Hypersensitivity reactions (including angioedema, anaphylactic reactions and anaphylactic shock)
Psychiatric disorders: Confusional state, Restlessness
Nervous System Disorders: Hemiplegia, Hypoesthesia, Seizures
Eye Disorders: Blepharospasm, Visual impairment, Vision blurred
Ear and Labyrinth Disorders: vertigo
Cardiac Disorders: Acute myocardial infarction, Coronary artery vasospasm, Angina pectoris, Tachycardia
Gastrointestinal Disorders: Abdominal discomfort, Abdominal pain, Abdominal pain upper, Colitis, Hypoesthesia oral, Swollen tongue
Skin and Subcutaneous Tissue Disorders: Cold sweat, Erythema, Hyperhidrosis
Musculoskeletal, Connective Tissue, and Bone Disorders: Arthralgia, Myalgia, Pain in extremity
Reproductive System and Breast Disorders: Breast pain
General Disorders: Malaise, Peripheral coldness.
3DESCRIPTION
Almotriptan malate tablets contain almotriptan malate, a selective 5-hydroxytryptamine
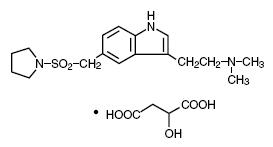
C
Almotriptan is a white to off white crystalline powder that is soluble in water. Almotriptan malate tablets for oral administration contain almotriptan malate equivalent to 6.25 mg or 12.5 mg of almotriptan. Each compressed tablet contains the following inactive ingredients: croscarmellose sodium, hypromellose, macrogol/PEG 8000, mannitol, microcrystalline cellulose, polydextrose FCC, povidone, sodium stearyl fumarate, titanium dioxide, and triacetin/glycerol triacetate.
4HOW SUPPLIED/STORAGE AND HANDLING
Almotriptan malate tablets are available as follows:
6.25 mg: White to off-white, round, convex, film-coated tablets debossed with “93” on one side and “A1” on the other side in boxes of 1 card X 6 tablets (NDC 0093-5260-18).
12.5 mg: White to off-white, round, convex, film-coated tablets debossed with “93” on one side and “A2” on the other side in boxes of 2 cards X 6 tablets (NDC 0093-5261-29).
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
5PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Drug Interactions
Advise patients to talk with their physician or pharmacist before taking any new medicines, including prescription and non-prescription drugs and supplements
Hypersensitivity
Inform patients to tell their physician if they develop a rash, itching, or breathing difficulties after taking almotriptan malate
Risk of Myocardial Ischemia and/or Infarction, Other Adverse Cardiac Events, Other Vasospasm-Related Events, and Cerebrovascular Events
Inform patients that almotriptan malate may cause serious cardiovascular side effects such as myocardial infarction or stroke, which may result in hospitalization and even death. Although serious cardiovascular events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative signs or symptoms. Apprise the patient of the importance of this follow-up
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome with the use of almotriptan malate or other triptans, particularly during combined use with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs)
Medication Overuse Headache
Inform patients that use of acute migraine drugs for 10 or more days per month may lead to an exacerbation of headache and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary)
Pregnancy
Advise patients to notify their physician if they become pregnant during treatment or intend to become pregnant
Nursing Mothers
Advise patients to notify their physician if they are breastfeeding or plan to breastfeed
Ability to Operate Machinery or Vehicles
Counsel patients that almotriptan malate may cause dizziness, somnolence, visual disturbances, and other CNS symptoms that can interfere with driving or operating machinery. Accordingly, advise the patient not to drive, operate complex machinery, or engage in other hazardous activities until they have gained sufficient experience with almotriptan malate to gauge whether it affects their mental or visual performance adversely.
Distributed By:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Rev. C 5/2017
6Package/Label Display Panel
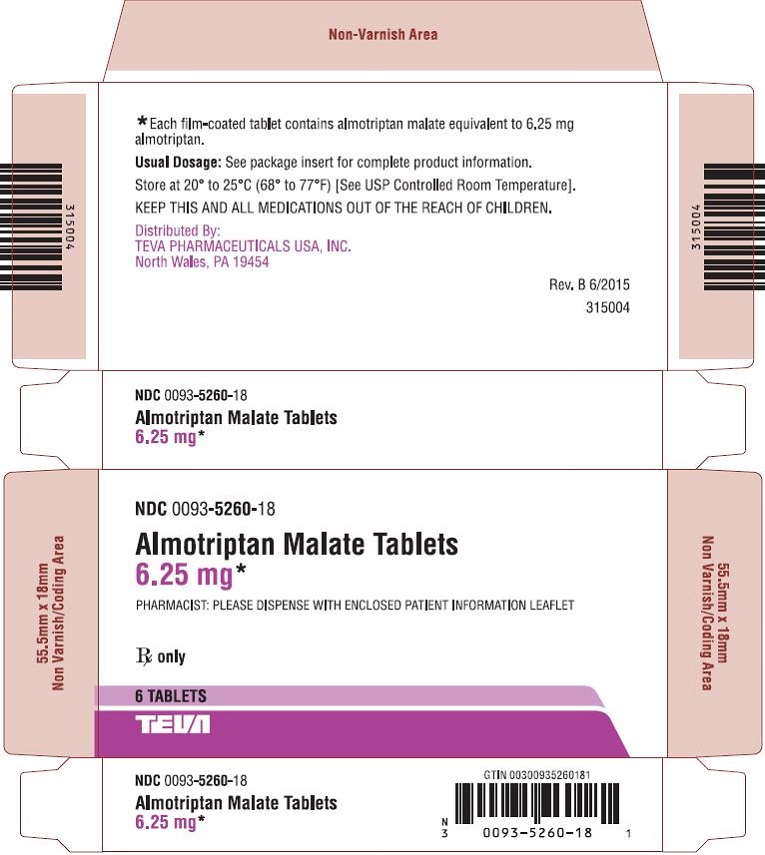
6.1Almotriptan Malate Tablets 6.25 mg, 6s Carton Text
NDC 0093-5260-18
Almotriptan Malate Tablets
6.25 mg*
PHARMACIST: PLEASE DISPENSE WITH ENCLOSED PATIENT INFORMATION LEAFLET
Rx only
6 TABLETS
TEVA
7Package/Label Display Panel
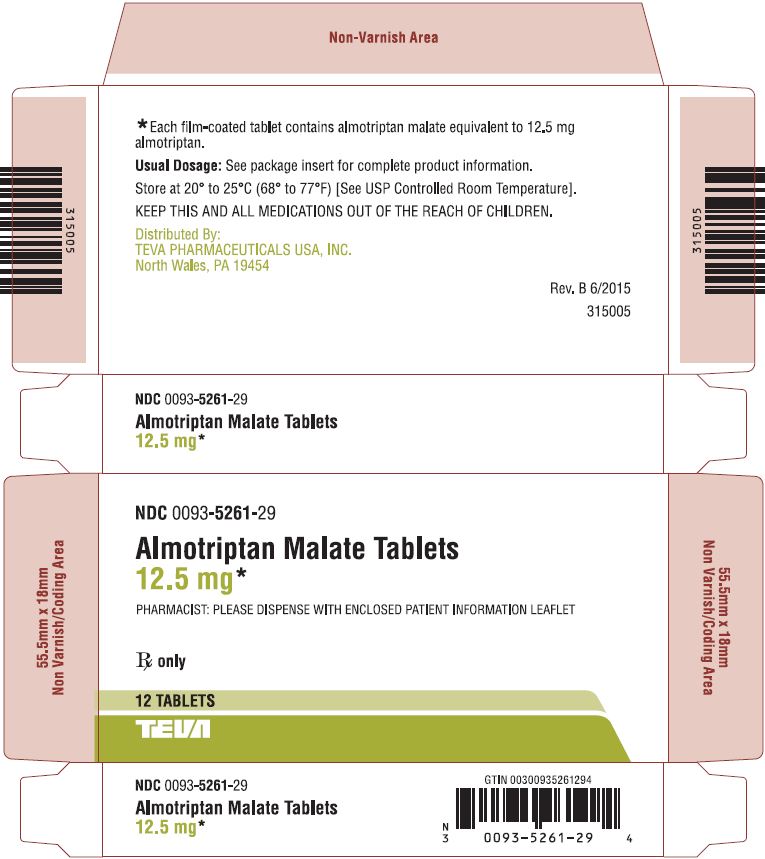
7.1Almotriptan Malate Tablets 12.5 mg, 12s Carton Text
NDC 0093-5261-29
Almotriptan Malate Tablets
12.5 mg*
PHARMACIST: PLEASE DISPENSE WITH ENCLOSED PATIENT INFORMATION LEAFLET
Rx only
12 TABLETS
TEVA
