Tremfya
What is Tremfya (Guselkumab)?
Living with moderate to severe plaque psoriasis or psoriatic arthritis can feel like a constant battle with your own body. The visible plaques, persistent itching, and painful joints can impact everything from your daily comfort to your self-confidence. For those who have tried other treatments without success, advanced therapies offer new hope. One such option is Tremfya (guselkumab), a prescription medication designed to target a specific source of inflammation driving these conditions.
Tremfya is part of a newer class of drugs known as biologics. Unlike traditional medications that are made from chemicals, biologics are complex proteins derived from living cells. They are designed to interact with specific parts of the immune system. Tremfya is not typically a first-line treatment; it is often prescribed when other therapies, such as topical treatments or oral medications, have not provided enough relief. Understanding how it works can empower you to have more informed conversations with your doctor and take an active role in your care.
What does Tremfya do?
Tremfya is approved by the U.S. Food and Drug Administration (FDA) to treat two related autoimmune conditions:
- Adults with moderate to severe plaque psoriasis who may benefit from systemic therapy (medications taken by injection or orally) or phototherapy (ultraviolet light treatment).
- Adults with active psoriatic arthritis, a condition that combines the skin symptoms of psoriasis with joint inflammation.
Patients taking Tremfya for plaque psoriasis can often expect a significant reduction in skin plaques, scaling, and itchiness. For those with psoriatic arthritis, the goal is to reduce joint pain, stiffness, and swelling, which can help improve physical function and prevent long-term joint damage.
Clinical studies have shown Tremfya to be highly effective. For example, in trials for plaque psoriasis, a large percentage of patients achieved 90% to 100% clearer skin after several months of treatment, a result that was often maintained with continued use (Janssen Biotech, Inc., 2020). This level of improvement can have a profound impact on a person’s quality of life.
How does Tremfya work?
To understand how Tremfya works, it helps to think of your immune system as a complex communication network. In autoimmune diseases like psoriasis, certain signals in this network become overactive, mistakenly telling the body to attack its own tissues. This leads to the rapid skin cell growth and inflammation that cause plaques and joint pain.
Tremfya works by targeting a very specific messenger protein in this network called interleukin-23 (IL-23). Think of IL-23 as a key conductor of an orchestra that is playing too loudly and out of tune. By precisely blocking IL-23, Tremfya quiets these specific inflammatory signals without suppressing the entire immune system.
This targeted approach is what sets it apart from some older, broader-acting medications. By interrupting this specific pathway, Tremfya helps calm the overactive immune response, leading to clearer skin and calmer joints. Its focused mechanism helps restore balance and control the underlying driver of the disease.
Tremfya side effects
Like all medications, Tremfya has potential side effects. Your healthcare provider will discuss these with you and monitor your health closely throughout treatment.
The most common side effects are generally mild to moderate and may include:
- Upper respiratory infections (like the common cold or sinus infections)
- Headache
- Injection site reactions (such as redness, pain, or swelling)
- Joint pain
- Diarrhea
- Stomach flu (gastroenteritis)
Because Tremfya affects the immune system, it may lower your ability to fight infections. Your doctor will screen you for tuberculosis (TB) before you start treatment and will monitor you for any signs of infection. It is important to tell your doctor if you develop a fever, cough, or other symptoms of an infection.
Serious side effects are rare but possible. Seek immediate medical attention if you experience signs of a serious allergic reaction, such as trouble breathing, hives, or swelling of your face, lips, tongue, or throat. People with an active, serious infection should not start Tremfya until the infection is treated (Mayo Clinic, 2024).
Tremfya dosage
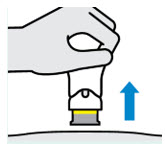
Tremfya is administered as an injection under the skin (subcutaneous injection). It is not a daily medication. After an initial “starter” dose, you will receive another dose four weeks later, followed by a maintenance dose every eight weeks.
Many patients learn to administer their injections at home after being trained by a healthcare professional. This can offer convenience and flexibility in managing your treatment schedule.
Before you begin, your doctor will perform a blood test to screen for tuberculosis. Throughout your treatment, they will continue to monitor you for any signs of infection or other side effects. Regular check-ins are a crucial part of the treatment plan to ensure Tremfya remains a safe and effective option for you.
Does Tremfya have a generic version?
Currently, there is no generic version of Tremfya. As a biologic medication, its complexity makes it difficult to replicate exactly.
The equivalent of a generic for a biologic drug is called a “biosimilar.” A biosimilar is a biologic product that is highly similar to, and has no clinically meaningful differences from, an existing FDA-approved biologic (FDA, 2023). At present, the FDA has not approved any biosimilars for Tremfya (guselkumab). Therefore, it is only available under its brand name.
Conclusion
Tremfya (guselkumab) represents a significant advancement in the treatment of moderate to severe plaque psoriasis and active psoriatic arthritis. By precisely targeting interleukin-23, it offers a way to control the underlying inflammation that drives these conditions, often leading to dramatic improvements in skin clarity and joint health.
While it comes with potential side effects, its safety profile is well-understood, and risks can be effectively managed through careful monitoring by your healthcare team. Every patient’s experience is unique, but for many, Tremfya provides a path toward lasting relief and a better quality of life. Partnering closely with your doctor will help you determine if this advanced therapy is the right choice for your health journey.
References
- Janssen Biotech, Inc. (2020). TEMPYA® (guselkumab) Prescribing Information. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761061s007lbl.pdf
- Mayo Clinic. (2024). Guselkumab (Subcutaneous Route). https://www.mayoclinic.org/drugs-supplements/guselkumab-subcutaneous-route/side-effects/drg-20406253
- U.S. Food and Drug Administration (FDA). (2023). Biosimilar and Interchangeable Biologics: https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to evaluate long-term safety of subcutaneous guselkumab in pediatric participants with moderately to severely active ulcerative colitis, or moderately to severely active Crohn's disease, or juvenile psoriatic arthritis (jPsA).
Summary: The purpose of this study is to evaluate the clinical and endoscopic efficacy of guselkumab in pediatric participants with Crohn's Disease (CD) at the end of maintenance therapy (Week 52) among participants who were in clinical response to guselkumab at Week 12.
Summary: The purpose of this study is to evaluate the efficacy of guselkumab in healing of all layers of the digestive tract (transmural healing) with the help of a score called Magnetic Resonance Index of Activity (MaRIA) based on a scan at Week 48.
Related Latest Advances
Brand Information
- Hypersensitivity Reactions
- Infections
- Tuberculosis
- Hepatotoxicity
200 mg/2mL (100 mg/mL) Prefilled Syringe
 Storage information
Storage information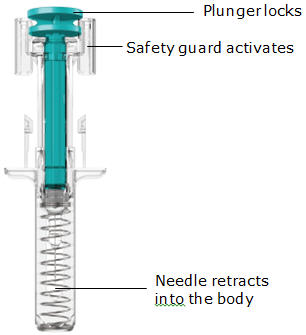

- cloudy or
- discolored or
- has large particles
- Front of thighs
- Lower stomach area (lower abdomen), except for a 2-inch area right around your navel (belly-button)
- Back of upper arms (only if someone else is giving you the injection)
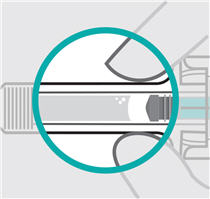
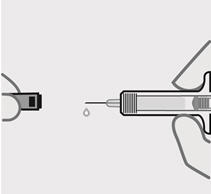
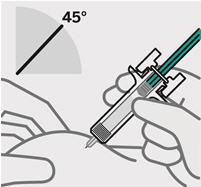
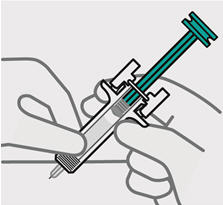
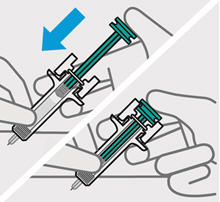
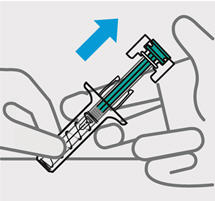
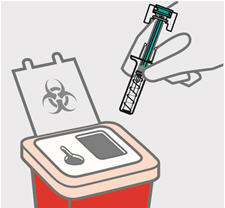
- made of heavy-duty plastic
- can be closed with a tight-fitting, puncture resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
 Need help?
Need help?[trem fye' ah Pen]
200 mg/2 mL Prefilled Pen
 Storage information
Storage information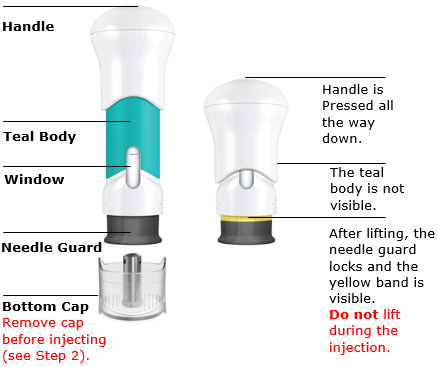

- cloudy or
- discolored or
- has large particles
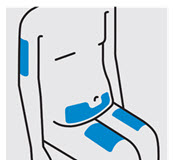
- Front of thighs
- Lower stomach area (lower abdomen), except for a 2-inch area right around your navel (belly-button)
- Back of upper arms (only if someone else is giving you the injection)
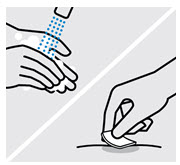
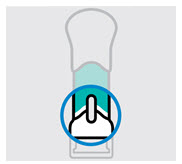
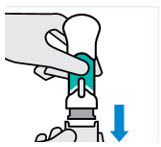

- made of heavy-duty plastic
- can be closed with a tight-fitting, puncture resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
 Need help?
Need help?(trem fye' ah)
(guselkumab)

 Storage information
Storage information



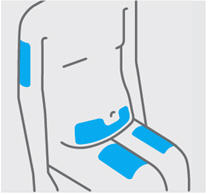
- Front of thighs (recommended)
- Lower stomach area (lower abdomen), except for a 2-inch area right around your navel (belly-button)
- Back of upper arms (only if someone else is giving you the injection)









 Need help?
Need help?- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
One-Press Patient-Controlled Injector

 Storage information
Storage information Need help?
Need help?



- Front of thighs (recommended)
- Lower stomach area (lower abdomen), except for a 2-inch area right around your navel (belly-button)
- Back of upper arms (only for adults and only if someone else is giving you the injection). This injection site is not for use in children.


- cloudy,
- discolored, or
- has large particles.



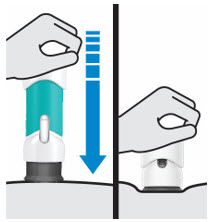
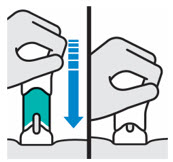
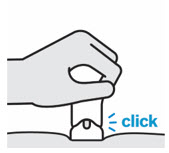
- The teal body is not visible.
- You cannot press the handle down anymore.
- You may hear a click.



- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
100 mg/mL Prefilled Pen




- cloudy or
- discolored or
- has large particles

- Front of thighs
- Lower stomach area (lower abdomen), except for a 2-inch area right around your navel (belly-button)
- Back of upper arms (only for adults and only if someone else is giving you the injection). This injection site is not for use in children.








- made of heavy-duty plastic
- can be closed with a tight-fitting, puncture resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container









