Brand Name
Horizant
Generic Name
Gabapentin Enacarbil
View Brand Information FDA approval date: May 01, 2013
Form: Tablet
What is Horizant (Gabapentin Enacarbil)?
HORIZANT is indicated for: treatment of moderate-to-severe primary Restless Legs Syndrome in adults.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Nighttime Agitation and Restless Legs Syndrome in People With Alzheimer's Disease
Summary: Nighttime agitation in persons with Alzheimer's disease causes patient suffering, distresses caregivers, and often results in prescriptions for harmful antipsychotics. Effective treatments are lacking because of limited knowledge of the etiology of nighttime agitation. The investigators propose a clinical trial to better elucidate whether a sleep disorder, restless legs syndrome, may be a mechanis...
Related Latest Advances
Brand Information
Horizant (gabapentin enacarbil)
1DOSAGE FORMS AND STRENGTHS
HORIZANT Extended-Release Tablets, 300 mg, are white to off-white, oval-shaped tablets debossed with "GS TF7" and 600 mg, are white to off-white, oval-shaped tablets debossed with "GS LFG". Both the 300 mg and 600 mg tablets may contain occasional black/grey spots.
2CONTRAINDICATIONS
None.
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described in more detail in the
- Effects on Driving
- Somnolence/Sedation and Dizziness
- Suicidal Behavior and Ideation
- Increased Risk of Seizures and Other Adverse Reactions with Abrupt or Rapid Discontinuation
- Respiratory Depression
- Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In all controlled and uncontrolled trials across various patient populations, more than 2,300 patients have received HORIZANT orally in daily doses ranging from 600 to 3,600 mg.
3.2Postmarketing Experience
The following adverse reactions have been reported in patients receiving gabapentin and have been identified during postapproval use of HORIZANT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: breast enlargement, gynecomastia, elevated creatine kinase, bullous pemphigoid.
There are postmarketing reports of life-threatening or fatal respiratory depression in patients taking gabapentin with opioids or other CNS depressants, or in the setting of underlying respiratory impairment [see Warnings and Precautions (
There are postmarketing reports of withdrawal symptoms after discontinuation of gabapentin. Reported adverse reactions include, but are not limited to, seizures, depression, suicidal ideation and behavior, agitation, confusion, disorientation, psychotic symptoms, anxiety, insomnia, nausea, pain, sweating, tremor, headache, dizziness, and malaise [see Warnings and Precautions (
4DRUG INTERACTIONS
Gabapentin enacarbil is released faster from HORIZANT Extended-Release tablets in the presence of alcohol. Consumption of alcohol is not recommended when taking HORIZANT
5DESCRIPTION
HORIZANT (gabapentin enacarbil) is a prodrug of gabapentin. Gabapentin enacarbil is described as (1-{[({(1
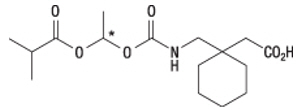
Gabapentin enacarbil is a white to off-white crystalline solid with a melting onset of approximately 64°C and a solubility of 0.5 mg/mL in water and 10.2 mg/mL in phosphate buffer (pH 6.3).
HORIZANT is administered orally. Each HORIZANT Extended-Release Tablet contains 300 mg or 600 mg of gabapentin enacarbil and the following inactive ingredients: colloidal silicon dioxide, dibasic calcium phosphate dihydrate, glyceryl behenate, magnesium stearate, sodium lauryl sulfate, and talc.
6HOW SUPPLIED/STORAGE AND HANDLING
HORIZANT Extended-Release Tablets containing 300 mg of gabapentin enacarbil are white to off-white, with occasional black/grey spots, oval-shaped tablets debossed with "GS TF7".
HORIZANT Extended-Release Tablets containing 600 mg of gabapentin enacarbil are white to off-white, with occasional black/grey spots, oval-shaped tablets debossed with "GS LFG". They are supplied as follows:
300 mg: NDC 53451-0103-1: Bottles of 30
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
8MEDICATION GUIDE
HORIZANT (ho-ri' zant)(gabapentin
Read this Medication Guide before you start taking HORIZANT and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about HORIZANT?
HORIZANT can cause serious side effects:
1. Do not drive after taking your dose of HORIZANT until you know how HORIZANT affects you, including the morning after you take your dose. Do notoperate heavy machinery or do other dangerous activities until you know how HORIZANT affects you. HORIZANT can cause sleepiness, dizziness, slow thinking, and can affect your coordination. Ask your healthcare provider when it would be okay to do these activities.
2. HORIZANT may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. This can happen while you take HORIZANT or after stopping HORIZANT.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempt to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated
- new or worse restlessness
- panic attacks
- new or worse trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop HORIZANT without first talking to a healthcare provider.Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
3. HORIZANT may cause a serious or life-threatening allergic reaction that may affect your skin or other parts of your body such as your liver or blood cells. You may or may not have rash with these types of reactions. Call a healthcare provider right away if you have any of the following symptoms:
- skin rash
- hives
- fever
- swollen glands that do not go away
- swelling of your lips or tongue
- yellowing of your skin or eyes
- unusual bruising or bleeding
- severe fatigue or weakness
- unexpected, severe muscle pain
- frequent infections
These symptoms may be the first signs of a serious reaction. A healthcare provider should examine you to decide if you should continue taking HORIZANT.
4. Serious breathing problems. Serious breathing problems can occur when HORIZANT is taken with other medicines that can cause severe sleepiness or decreased awareness, or when it is taken by someone who already has breathing problems. Watch for increased sleepiness or decreased breathing when starting HORIZANT or when the dose is increased. Get help right away if breathing problems occur.
What is HORIZANT?
HORIZANT is a prescription medicine used to treat adults with:
- moderate-to-severe primary Restless Legs Syndrome (RLS).
- pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection).
HORIZANT is not for people with RLS who need to sleep during the daytime and need to stay awake at night.
HORIZANT is not the same medicine as gabapentin (for example, NEURONTIN
It is not known if HORIZANT is safe and effective in children.
What should I tell my healthcare provider before taking HORIZANT?
Before taking HORIZANT, tell your healthcare provider if you:
- have or have had kidney problems or are on hemodialysis.
- have or have had depression, mood problems, or suicidal thoughts or behavior.
- have or have had seizures.
- have a history of drug abuse.
- have breathing problems.
- have any other medical conditions.
- are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. Your body turns HORIZANT into another drug (gabapentin) that passes into your milk. It is not known if this can harm your baby. You and your healthcare provider should decide if you will take HORIZANT or breastfeed.
- drink alcohol.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take any opioid pain medicine (such as oxycodone), any medicines for anxiety (such as lorazepam) or insomnia (such as zolpidem), or any medicines that make you sleepy.
You may have a higher chance for dizziness, sleepiness, or breathing problems if these medicines are taken with HORIZANT.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take HORIZANT?
- Take HORIZANT exactly as your healthcare provider tells you to take it. Your healthcare provider will tell you how much HORIZANT to take and when to take it.
- Take HORIZANT tablets whole.
- Take HORIZANT tablets with food.
- Do not stop taking HORIZANT without talking to your healthcare provider first.If you stop taking HORIZANT suddenly, you may develop side effects.
- If you forget to take your medicine at the time recommended by your healthcare provider, just skip the missed dose. Take the next dose at your regular time.
- If you take too much HORIZANT, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking HORIZANT?
- Do not take other medicines that make you sleepy or dizzy while taking HORIZANT without first talking with your healthcare provider. Taking HORIZANT with medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not take other gabapentin drugs (for example, NEURONTIN or GRALISE) while you take HORIZANT.
- Do not consume alcohol when taking HORIZANT.
What are the possible side effects of HORIZANT?
- See
The most common side effects of HORIZANT include:
- sleepiness
- dizziness
- headache
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of HORIZANT. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store HORIZANT?
- Store HORIZANT between 59° and 86°F (15° and 30°C).
- Keep HORIZANT dry and away from moisture.
- Keep HORIZANT tightly closed in the bottle provided to you. Do not remove any moisture control packs that may come in the bottle.
Keep HORIZANT and all medicines out of the reach of children.
General Information about the safe and effective use of HORIZANT
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use HORIZANT for a condition for which it was not prescribed. Do not give HORIZANT to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about HORIZANT. If you would like more information, talk with your healthcare provider.
You can ask your healthcare provider or pharmacist for information about HORIZANT that was written for healthcare professionals.
For more information about HORIZANT, go to www.HORIZANT.com or call 1-800-461-7449.
What are the ingredients in HORIZANT?
Active ingredients:gabapentin enacarbil
Inactive ingredients:Both the 300 mg and 600 mg tablets contain colloidal silicon dioxide, dibasic calcium phosphate dihydrate, glyceryl behenate, magnesium stearate, sodium lauryl sulfate, and talc.
Manufactured for:
Azurity Pharmaceuticals, Inc.
HORIZANT is a registered trademark of Azurity Pharmaceuticals, Inc. The other brands listed are trademarks of their respective owners and are not trademarks of Azurity Pharmaceuticals, Inc. The makers of these brands are not affiliated with and do not endorse Azurity Pharmaceuticals, Inc. or its products.
HZT-MG-03 Rev. 04/2025
This Medication Guide has been approved by the U.S. Food and Drug Administration.
9PRINCIPAL DISPLAY PANEL - 300 mg Tablet Bottle Label
30 Tablets
Horizant
300 mg
Dispense the accompanying
Rx only

10PRINCIPAL DISPLAY PANEL - 600 mg Tablet Bottle Label
30 Tablets
Horizant
600 mg
Dispense the accompanying
Rx only
azurity
HZT-TL600-02

