Braftovi
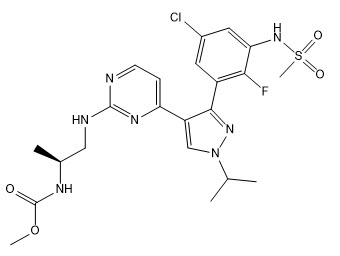
What is Braftovi (Encorafenib)?
Top Global Experts
Related Clinical Trials
Summary: The purpose of this study is to assess rate of disease relapse and hazard rate of disease relapse after neoadjuvant therapy based on the statuses of pathologic complete response or non-pathologic complete response, and postoperative adjuvant therapy.
Summary: The goal of this interventional clinical trial is to provide proof-of-principle data for the biologic activity of defactinib in combination with avutometinib in brain metastases from melanoma, and to define the potential role of the combination with mutant BRAF inhibitors or after BRAF/MEK inhibitors in BRAF V600E/K mutant tumors, in individuals with advanced melanoma who experience the developmen...
Summary: The purpose of this clinical trial is to learn the safety and effects of the study medicine (PF-07799544) administered as a single agent and in combination with other study medications in people with solid tumors. This study is seeking participants who have an advanced solid tumor for which the available treatments are no longer effective in controlling their cancer. All participants in this study...
Related Latest Advances
Brand Information
- New Primary Malignancies
- Tumor Promotion in BRAF Wild-Type Tumors
- Cardiomyopathy
- Hepatotoxicity
- Hemorrhage
- Uveitis
- QT Prolongation
- Embryo-Fetal Toxicity
- Risks Associated with BRAFTOVI as a Single Agent
- Risks Associated with Combination Treatment