Generic Name
Lamivudine
Brand Names
Abacavir, Efavirenz, Cimduo, Epzicom, Epivir, Delstrigo, SYMFI, Combivir, Triumeq, Dovato
FDA approval date: October 04, 2010
Classification: Human Immunodeficiency Virus Nucleoside Analog Reverse Transcriptase Inhibitor
Form: Tablet, Kit, Solution
What is Abacavir (Lamivudine)?
Efavirenz, lamivudine and tenofovir disoproxil fumarate tablets are indicated as a complete regimen for the treatment of human immunodeficiency virus type 1 infection in adult and pediatric patients weighing at least 35 kg. Efavirenz, lamivudine and tenofovir disoproxil fumarate tablets are three-drug combination of efavirenz , a non-nucleoside reverse transcriptase inhibitor, and lamivudine and tenofovir disoproxil fumarate , both nucleoside reverse transcriptase inhibitors and are indicated as a complete regimen for the treatment of human immunodeficiency virus type 1 infection in adult and pediatric patients weighing at least 35 kg.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Abacavir and Lamivudine (Abacavir and Lamivudine)
1OVERDOSAGE
There is no known specific treatment for overdose with abacavir and lamivudine tablets. If overdose occurs, the patient should be monitored, and standard supportive treatment applied as required.
Abacavir
It is not known whether abacavir can be removed by peritoneal dialysis or hemodialysis.
Lamivudine
Because a negligible amount of lamivudine was removed via (4-hour) hemodialysis, continuous ambulatory peritoneal dialysis, and automated peritoneal dialysis, it is not known if continuous hemodialysis would provide clinical benefit in a lamivudine overdose event.
2DESCRIPTION
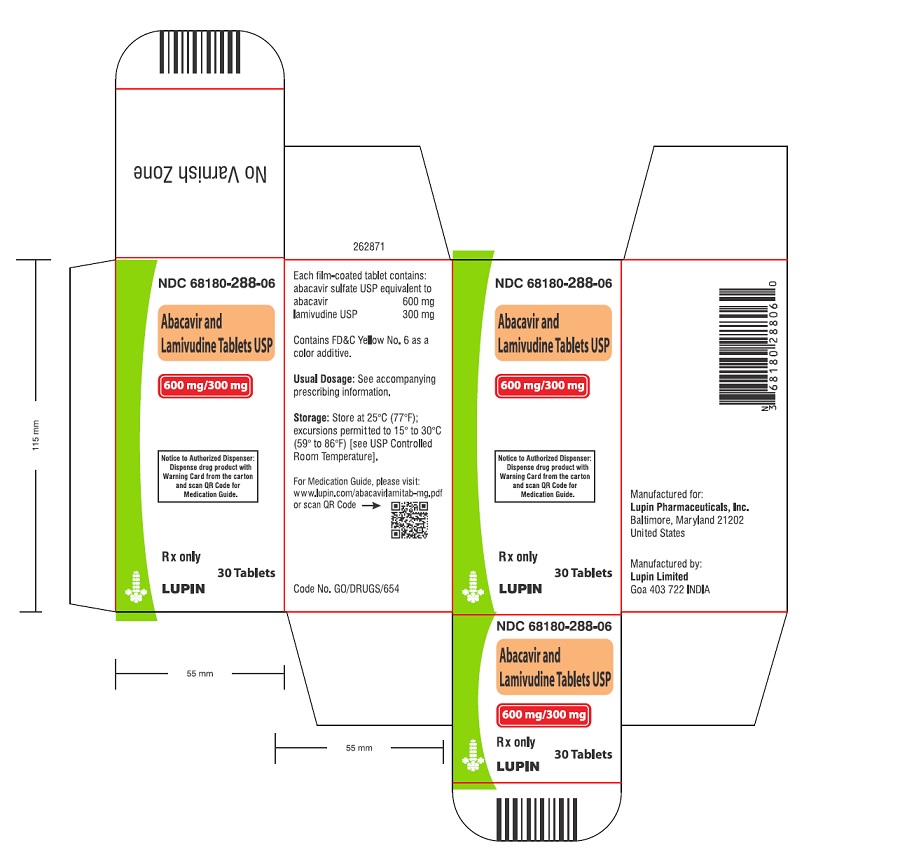
Abacavir and Lamivudine Tablets USP
Abacavir and lamivudine tablets USP contain the following 2 synthetic nucleoside analogues: abacavir (ZIAGEN, also a component of TRIZIVIR
Abacavir and lamivudine tablets USP are for oral administration. Each orange colored, oval shaped, biconvex, film-coated tablet contains the active ingredients 600 mg of abacavir as abacavir sulfate and 300 mg of lamivudine, and the inactive ingredients crospovidone, magnesium stearate, microcrystalline cellulose, and povidone. The tablets are coated with a film that is made of FD&C Yellow No. 6, hypromellose, polyethylene glycol, polysorbate, and titanium dioxide.
Abacavir Sulfate
The chemical name of abacavir sulfate is
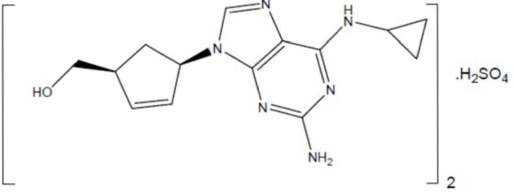
Abacavir sulfate is a white to off-white solid with a solubility of approximately 77 mg per mL in distilled water at 25°C.
In vivo, abacavir sulfate dissociates to its free base, abacavir. Dosages are expressed in terms of abacavir.
Lamivudine
The chemical name of lamivudine is (2R, cis)-4-amino-1-(2- hydroxymethyl-1,3-oxathiolan-5-yl)-(1H)-pyrimidin-2-one. Lamivudine is the (-) enantiomer of a dideoxy analogue of cytidine. Lamivudine has also been referred to as (-)2',3'-dideoxy, 3'thiacytidine. It has a molecular formula of C
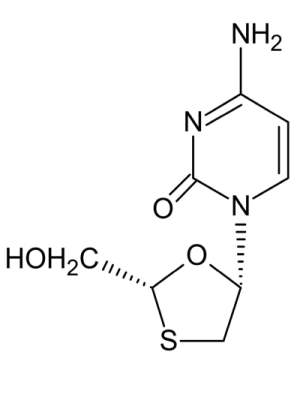
Lamivudine is a white to off-white solid with a solubility of approximately 68 mg per mL in water at 20°C.
3HOW SUPPLIED/STORAGE AND HANDLING
Abacavir and lamivudine tablets USP are available as tablets. Each tablet contains 600 mg of abacavir as abacavir sulfate and 300 mg of lamivudine. The tablets are orange colored, oval shaped, biconvex, film-coated tablets debossed with "LU" on one side "C51" on the other side. They are packaged as follows:
Bottles (120cc) of 30 Tablets (NDC 68180-288-06).
Bottles (250cc) of 90 Tablets (NDC 68180-288-09).
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
4PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Hypersensitivity Reactions
Inform patients:
- that a Medication Guide and Warning Card summarizing the symptoms of the abacavir hypersensitivity reaction and other product information will be dispensed by the pharmacist with each new prescription and refill of abacavir and lamivudine tablets, and instruct the patient to read the Medication Guide and Warning Card every time to obtain any new information that may be present about abacavir and lamivudine tablets. The complete text of the Medication Guide is reprinted at the end of this document.
- to carry the Warning Card with them.
- how to identify a hypersensitivity reaction
- that if they develop symptoms consistent with a hypersensitivity reaction they should call their healthcare provider right away to determine if they should stop taking abacavir and lamivudine tablets.
- that a hypersensitivity reaction can worsen and lead to hospitalization or death if abacavir and lamivudine tablets is not immediately discontinued.
- to not restart abacavir and lamivudine tablets or any other abacavir-containing product following a hypersensitivity reaction because more severe symptoms can occur within hours and may include life-threatening hypotension and death.
- that if they have a hypersensitivity reaction, they should dispose of any unused abacavir and lamivudine tablets to avoid restarting abacavir.
- that a hypersensitivity reaction is usually reversible if it is detected promptly and abacavir and lamivudine tablets is stopped right away.
- that if they have interrupted abacavir and lamivudine tablets for reasons other than symptoms of hypersensitivity (for example, those who have an interruption in drug supply), a serious or fatal hypersensitivity reaction may occur with reintroduction of abacavir.
- to not restart abacavir and lamivudine tablets or any other abacavir-containing product without medical consultation and only if medical care can be readily accessed by the patient or others.
Patients with Hepatitis B or C Co-infection
Advise patients co-infected with HIV-1 and HBV that worsening of liver disease has occurred in some cases when treatment with lamivudine was discontinued. Advise patients to discuss any changes in regimen with their physician
Lactic Acidosis/Hepatomegaly with Steatosis
Advise patients that lactic acidosis and severe hepatomegaly with steatosis have been reported with use of nucleoside analogues and other antiretrovirals. Advise patients to stop taking abacavir and lamivudine tablets if they develop clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity
Immune Reconstitution Syndrome
Advise patients to inform their healthcare provider immediately of any signs and symptoms of infection as inflammation from previous infection may occur soon after combination antiretroviral therapy, including when abacavir and lamivudine tablet is started
Redistribution/Accumulation of Body Fat
Inform patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to abacavir and lamivudine tablets during pregnancy
Lactation
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in the breast milk
Missed Dose
Instruct patients that if they miss a dose of abacavir and lamivudine tablets, to take it as soon as they remember. Advise patients not to double their next dose or take more than the prescribed dose
Availability of Medication Guide
Instruct patients to read the Medication Guide before starting abacavir and lamivudine tablets and to re-read it each time the prescription is renewed. Instruct patients to inform their physician or pharmacist if they develop any unusual symptom, or if any known symptom persists or worsens.
The other brands listed are trademarks of their respective owners and are not trademarks of Lupin Pharmaceuticals, Inc. The makers of these brands are not affiliated with and do not endorse Lupin Pharmaceuticals, Inc. or its products.
5PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Abacavir and Lamivudine Tablets USP
Rx Only
600 mg and 300 mg
NDC 68180-288-06
30 Tablets
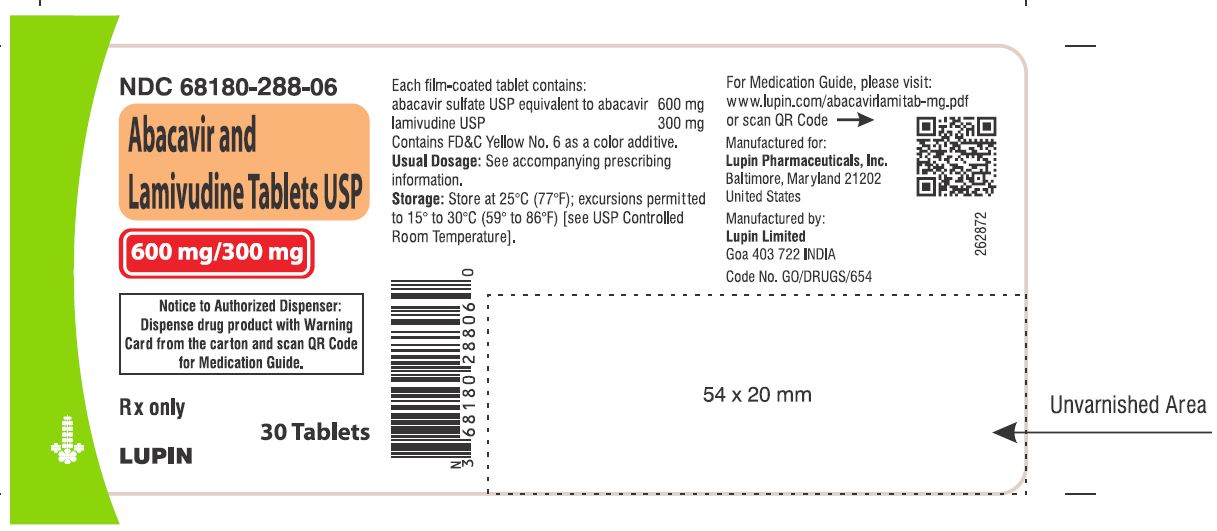
Abacavir and Lamivudine Tablets USP
Rx Only
600 mg and 300 mg
NDC 68180-288-06
30 Tablets