Brand Name
Ecoza
Generic Name
Econazole
View Brand Information FDA approval date: November 26, 2002
Classification: Azole Antifungal
Form: Cream, Aerosol
What is Ecoza (Econazole)?
Econazole Nitrate Cream, 1% is indicated for topical application in the treatment of tinea pedis, tinea cruris, and tinea corporis caused by Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans, Microsporum canis, Microsporum audouini, Microsporum gypseum, and Epidermophyton floccosum, in the treatment of cutaneous candidiasis, and in the treatment of tinea versicolor.
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Ecoza (econazole nitrate)
1INDICATIONS AND USAGE
Ecoza (econazole nitrate) topical foam, 1%, is indicated for the treatment of interdigital tinea pedis caused by
2DOSAGE AND ADMINISTRATION
Ecoza topical foam, 1% is for topical use only. Ecoza topical foam, 1% is not for oral, ophthalmic, or intravaginal use.
Ecoza topical foam, 1% should be applied to cover affected areas once daily for 4 weeks.
3DOSAGE FORMS AND STRENGTHS
Foam, 1%. Each gram of Ecoza topical foam, 1%, contains 10 mg of econazole nitrate in a white to off-white foam.
4CONTRAINDICATIONS
None.
5DESCRIPTION
Ecoza (econazole nitrate) topical foam, 1% contains the azole antifungal agent, econazole nitrate in an oil-in-water emulsion base consisting of the following inactive ingredients: dimethicone, glycerin, polysorbate 20, povidone, propylene glycol, stearic acid, trolamine, purified water and butane as a propellant. Each gram of Ecoza topical foam, 1% contains 10 mg of econazole nitrate, USP, in a white to off-white foam. Ecoza topical foam, 1% is alcohol (ethanol)-free and for topical use only.
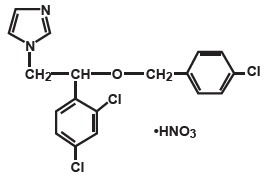
Chemically, econazole nitrate is 1-[2-{(4-chloro-phenyl)methoxy}-2-(2,4-dichlorophenyl) ethyl]-1H-imidazole mononitrate. Econazole nitrate has the molecular formula C
6CLINICAL STUDIES
In two multi-center, randomized, double-blind, vehicle-controlled clinical trials a total of 505 subjects with interdigital tinea pedis were randomized 1:1 to Ecoza topical foam or vehicle; subjects applied the assigned medication once daily for 4 weeks. The severity of erythema, scaling, fissuring, maceration, vesiculation, and pruritus were graded using a 4-point scale (none, mild, moderate, severe). Subjects had KOH examination and fungal cultures taken to confirm eligibility. A total of 339 subjects with positive fungal cultures were evaluated for efficacy. Efficacy was evaluated on Day 43, 2 weeks post-treatment with treatment success being defined as complete cure (negative KOH and fungal culture and no evidence of clinical disease). The study population ranged in age from 12 to 71 years with 5 subjects less than 18 years of age at baseline. The subjects were 71% male and 51% Caucasian. Table 1 presents the efficacy results for each trial.
a Mycological cure and an absence of clinical signs and symptoms (erythema, scaling, fissuring, maceration, vesiculation, or pruritus).
b Mycological cure and no or mild erythema and/or scaling with all other signs and symptoms absent.
c Negative KOH and fungal culture.
b Mycological cure and no or mild erythema and/or scaling with all other signs and symptoms absent.
c Negative KOH and fungal culture.
7HOW SUPPLIED/ STORAGE AND HANDLING
Ecoza topical foam, 1% is white to off-white foam supplied in 10g (NDC 81811-100-10) and 70g (NDC 81811-100-70) aluminum pressurized canister.
8PATIENT COUNSELING INFORMATION
See
The patient should be instructed as follows:
- Inform patients that Ecoza (econazole nitrate) topical foam, 1% is for topical use only. Ecoza (econazole nitrate) topical foam, 1% is not intended for oral, intravaginal, or ophthalmic use.
- Ecoza topical foam, 1% is flammable; avoid heat, flame, and smoking during and immediately following application.
- If a reaction suggesting sensitivity or chemical irritation develops with the use of Ecoza topical foam, 1%, use of the medication should be discontinued.
9Instructions for Use ECOZA ®(ee-ko-zah) (econazole nitrate) topical foam, 1%
Parts of Ecoza topical foam Canister. (See
How to apply Ecoza topical foam:
How should I store Ecoza topical foam?
- Store Ecoza topical foam at room temperature, between 68°F to 77°F (20°C to 25°C).
- Do not refrigerate or freeze Ecoza topical foam.
- Do not store Ecoza topical foam in direct sunlight.
- Ecoza topical foam is flammable. Keep the Ecoza topical foam canister away from heat and temperatures above 120°F (49°C), even if the canister is empty.
- Do not puncture or burn the Ecoza topical foam canister.
Keep Ecoza topical foam and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for Resilia Pharmaceuticals, Inc., Atlanta, GA 30328
Issued: 12/2023
10PRINCIPAL DISPLAY PANEL - 70 g Canister Label
NDC 81811-100-70
ecoza™
For Topical Use Only
Not for ophthalmic, oral or intravaginal use.
Keep Out of Reach of Children
Rx Only
Resilia Pharmaceuticals, Inc.

11PRINCIPAL DISPLAY PANEL - 70 g Canister Carton
NDC 81811-0100-70
ecoza™
For Topical Use Only
Not for ophthalmic, oral or intravaginal use.
Keep Out of Reach of Children
Rx Only
Resilia Pharmaceuticals, Inc.
