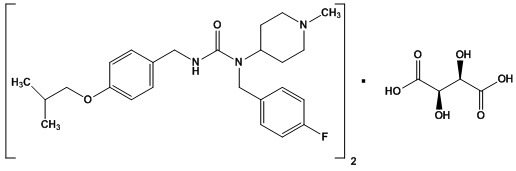
Nuplazid
What is Nuplazid (Pimavanserin)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This Phase 2 study examines the safety, tolerability, and preliminary efficacy of pimavanserin in individuals with Autism Spectrum Disorder. Male or female participants aged 16 to 40 years of age will be randomized to receive single doses of either placebo or pimavanserin in this randomized, placebo-controlled, cross-over designed study, followed by open label extension.
Summary: The primary objective of this study is to determine whether treatment with pimavanserin or quetiapine is associated with a greater improvement in psychosis when used in a routine clinical setting to treat hallucinations and/or delusions due to Parkinson's disease (PD) or dementia with Lewy bodies (DLB) - collectively referred to as Lewy body disease (LBD).
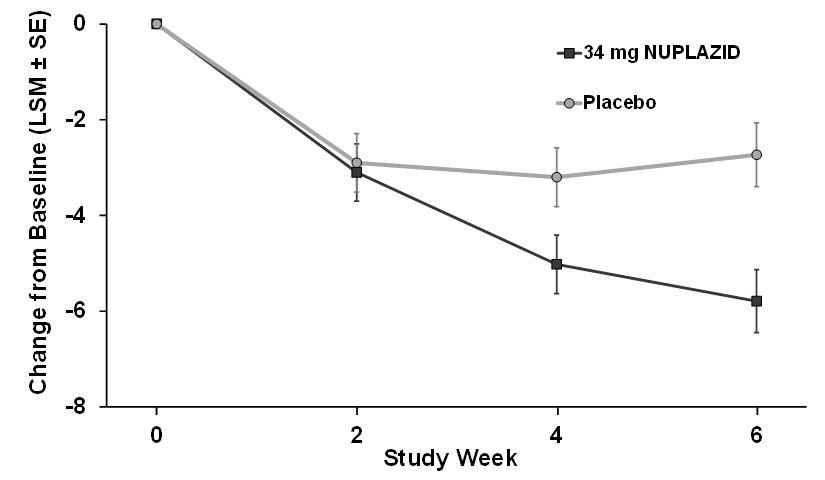
Summary: Patients with Parkinson's disease (PD) sometimes experience symptoms affecting their movement, such as slowness, tremor, stiffness, and balance or walking problems. Many patients also have other symptoms not related to movement, called non-motor symptoms, which may affect one's mood or emotions, memory or thinking, or cause one to see or hear things that aren't real (hallucinations) or believe thi...
Related Latest Advances
Brand Information
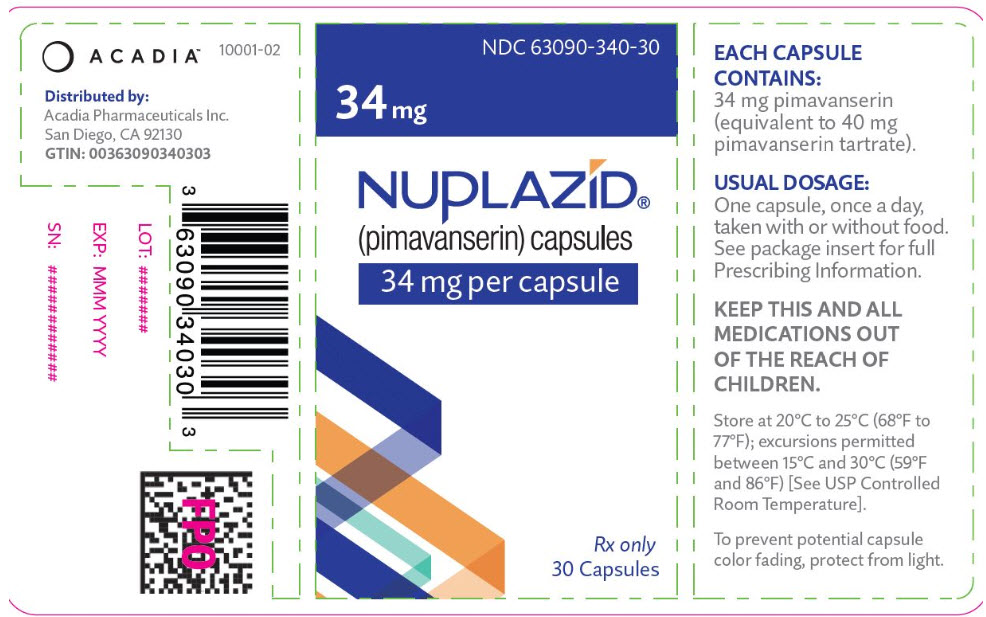
- 34 mg strength capsules. The capsules are opaque white and light green with "PIMA" and "34" printed in black.
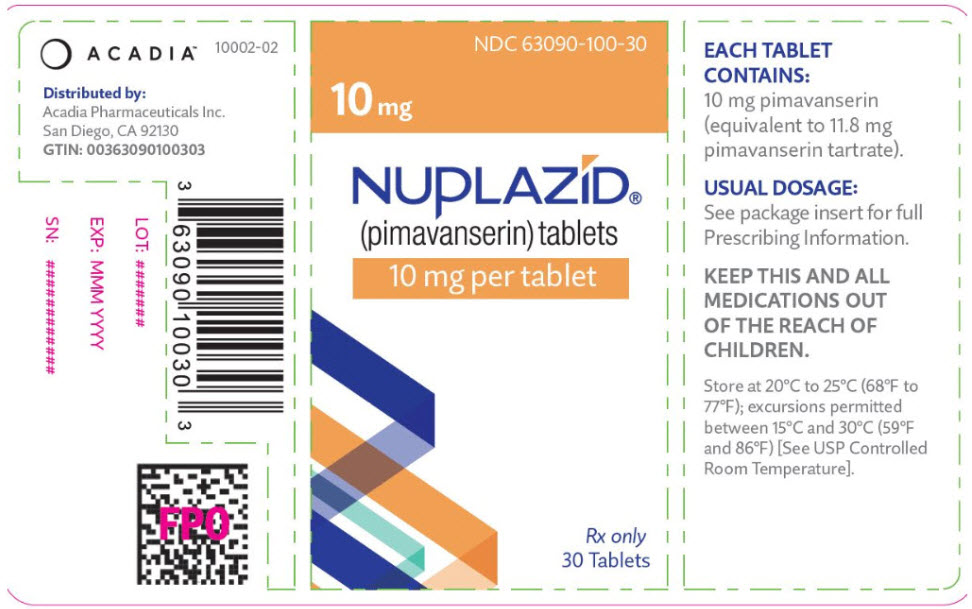
- 10 mg strength tablets. The orange, round, coated tablets are debossed on one side with a "P" and "10" on the reverse side.
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis
- QT Interval Prolongation