Generic Name
TC99M Sestamibi
Brand Names
99M Sestamibi, Cardiolite
FDA approval date: December 20, 1990
Classification: Radioactive Diagnostic Agent
Form: Injection
What is 99M Sestamibi (TC99M Sestamibi)?
Technetium Tc99m Sestamibi is a myocardial perfusion agent indicated for: detecting coronary artery disease by localizing myocardial ischemia and infarction evaluating myocardial function and developing information for use in patient management decisions Myocardial Imaging: Technetium Tc99m Sestamibi is a myocardial perfusion agent that is indicated for detecting coronary artery disease by localizing myocardial ischemia and infarction , in evaluating myocardial function and developing information for use in patient management decisions. Technetium Tc99m Sestamibi evaluation of myocardial ischemia can be accomplished with rest and cardiovascular stress techniques . It is usually not possible to determine the age of a myocardial infarction or to differentiate a recent myocardial infarction from ischemia. Breast Imaging: Technetium Tc99m Sestamibi is indicated for planar imaging as a second line diagnostic drug after mammography to assist in the evaluation of breast lesions in patients with an abnormal mammogram or a palpable breast mass. Technetium Tc99m Sestamibi is not indicated for breast cancer screening, to confirm the presence or absence of malignancy, and it is not an alternative to biopsy.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
TECHNETIUM TC 99M SESTAMIBI (Technetium TC 99m Sestamibi)
1INDICATIONS AND USAGE
Myocardial Imaging: Kit for the preparation of Technetium Tc 99m Sestamibi Injection is a myocardial perfusion agent that is indicated for detecting coronary artery disease by localizing myocardial ischemia (reversible defects) and infarction (non-reversible defects), in evaluating myocardial function and developing information for use in patient management decisions. Technetium Tc 99m Sestamibi evaluation of myocardial ischemia can be accomplished with rest and cardiovascular stress techniques (e.g., exercise or pharmacologic stress in accordance with the pharmacologic stress agents labeling).
It is usually not possible to determine the age of a myocardial infarction or to differentiate a recent myocardial infarction from ischemia.
Breast Imaging: Kit for the preparation of Technetium Tc 99m Sestamibi Injection is indicated for planar imaging as a second line diagnostic drug after mammography to assist in the evaluation of breast lesions in patients with an abnormal mammogram or a palpable breast mass.
Kit for the preparation of Technetium Tc 99m Sestamibi Injection is not indicated for breast cancer screening, to confirm the presence or absence of malignancy, and it is not an alternative to biopsy.
2DOSAGE AND ADMINISTRATION
For Myocardial Imaging: The suggested dose range for I.V. administration of Technetium Tc 99m Sestamibi in a single dose to be employed in the average patient (70 kg) is 370 to 1110 MBq (10 to 30 mCi).
For Breast Imaging: The recommended dose range for I.V. administration of Technetium Tc 99m Sestamibi is a single dose of 740 to 1110 MBq (20 to 30 mCi).
2.1Image Acquisition
Breast Imaging: It is recommended that images are obtained with a table overlay to separate breast tissue from the myocardium and liver, and to exclude potential activity that may be present in the opposite breast. For lateral images, position the patient prone with the isolateral arm comfortably above the head, shoulders flat against the table, head turned to the side and relaxed, with the breast imaged pendent through an overlay cutout. The breast should not be compressed on the overlay. For anterior images, position the patient supine with both arms behind the head. For either lateral or anterior images, shield the chest and abdominal organs, or remove them from the field of view.
For complete study, sets of images should be obtained five minutes after the injection, and in the following sequence:
Beginning five minutes after the injection of Technetium Tc 99m Sestamibi:
- ten-minute lateral image of breast with abnormality
- ten-minute lateral image of contralateral breast
- ten-minute anterior image of both breasts
2.2Radiation Dosimetry
The radiation doses to organs and tissues of an average patient (70 kg) per 1110 MBq (30 mCi) of Technetium Tc 99m Sestamibi injected intravenously are shown in
2.3Instructions for Preparation
Preparation of the Technetium Tc 99m Sestamibi from the Kit for the Preparation of Technetium Tc 99m Sestamibi is done by the following aseptic procedure:
General Procedure:
a. Prior to adding the Sodium Pertechnetate Tc 99m Injection to the vial, inspect the vial carefully for the presence of damage, particularly cracks, and do not use the vial if found. Tear off a radiation symbol and attach it to the neck of the vial.
b. Waterproof gloves should be worn during the preparation procedure. Remove the plastic disc from the vial and swab the top of the vial closure with alcohol to sanitize the surface.
Boiling Water Bath Procedure:
c. Place the vial in a suitable radiation shield with a fitted radiation cap.
d. With a sterile shielded syringe, aseptically obtain additive-free, sterile, non-pyrogenic Sodium Pertechnetate Tc 99m Injection [925 to 5550 MBq, (25 to 150 mCi)] in approximately 1 to 3 mL.
e. Aseptically add the Sodium Pertechnetate Tc 99m Injection to the vial in the lead shield. Without withdrawing the needle, remove an equal volume of headspace to maintain atmospheric pressure within the vial.
f. Shake vigorously, about 5 to 10 quick upward-downward motions.
g. Remove the vial from the lead shield and place upright in an appropriately shielded and contained boiling water bath, such that the vial is suspended above the bottom of the bath, and boil for 10 minutes. Timing for 10 minutes is begun as soon as the water begins to boil again. Do not allow the boiling water to come in contact with the aluminum crimp.
h. Remove the vial from the water bath, place in the lead shield and allow to cool for fifteen (15) minutes.
i. Using proper shielding, the vial contents should be visually inspected. Use only if the solution is clear and free of particulate matter and discoloration.
j. Assay the reaction vial using a suitable radioactivity calibration system. Record the Technetium Tc 99m concentration, total volume, assay time and date, expiration time and lot number on the radioassay information label and affix the label to the shield.
k. Store the reaction vial containing the Technetium Tc 99m Sestamibi at 15 °C to 25 °C (59 °F to 77 °F) until use; at such time the product should be aseptically withdrawn. Technetium Tc 99m Sestamibi should be used within six (6) hours of preparation. The vial contains no preservative.
Note: Adherence to the above product reconstitution instructions is recommended.
The potential for cracking and significant contamination exists whenever vials containing radioactive material are heated.
This product is not to be used with the Recon-o-stat (thermal cycler) due to smaller vial size requirements of this heating device.
Product should be used within six (6) hours after preparation.
Final product with radiochemical purity of at least 90% was used in the clinical trials that established safety and effectiveness. The radiochemical purity was determined by the following method.
The potential for cracking and significant contamination exists whenever vials containing radioactive material are heated.
This product is not to be used with the Recon-o-stat (thermal cycler) due to smaller vial size requirements of this heating device.
Product should be used within six (6) hours after preparation.
Final product with radiochemical purity of at least 90% was used in the clinical trials that established safety and effectiveness. The radiochemical purity was determined by the following method.
2.4Determination of Radiochemical Purity in Technetium Tc 99m Sestamibi
- Obtain a Baker-Flex Aluminum Oxide coated, plastic TLC plate, #1 B-F, pre-cut to 2.5cm x 7.5cm.
- Dry the plate or plates at 100°C for 1 hour and store in a desiccator. Remove pre-dried plate from the desiccator just prior to use.
- Apply 1 drop of ethanol* using a 1 mL syringe with a 22 to 26 gauge needle, 1.5cm from the bottom of the plate. THE SPOT SHOULD NOT BE ALLOWED TO DRY.
- Add 2 drops of Technetium Tc 99m Sestamibi solution, side by side on top of the ethanol* spot. Return the plate to a desiccator and allow the sample spot to dry (typically 15 minutes).
- The TLC tank is prepared by pouring ethanol* to a depth of 3 to 4 mm. Cover the tank and let it equilibrate for ~10 minutes.
- Develop the plate in the covered TLC tank in ethanol* for a distance of 5 cm from the point of application.
- Cut the TLC plate 4 cm from the bottom and measure the Tc 99m activity in each piece by appropriate radiation detector.
- Calculate the % Tc 99m Sestamibi as:
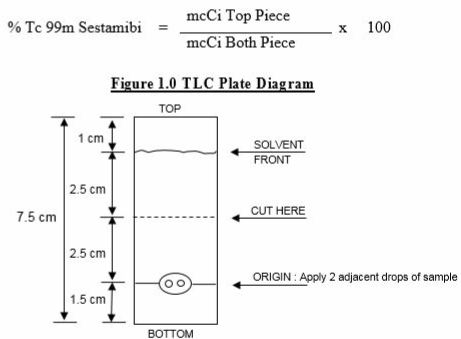
*The ethanol used in this procedure should be 95% or greater. Absolute ethanol (99%) should remain at ≥ 95% ethanol content for one week after opening if stored tightly capped, in a cool dry place.
3DOSAGE FORMS AND STRENGTHS
Kit for the Preparation of Technetium Tc 99m Sestamibi Injection is supplied as a lyophilized sterile and non-pyrogenic mixture in a 10 mL vial.
4CONTRAINDICATIONS
None known.
5ADVERSE REACTIONS
Adverse events were evaluated in 3741 adults who were evaluated in clinical studies. Of these patients, 3068 (77% men, 22% women, and 0.7 % of the patients' genders were not recorded) were in cardiac clinical trials and 673 (100% women) in breast imaging trials. Cases of angina, chest pain, and death have occurred [
In the clinical studies for breast imaging, breast pain was reported in 12 (1.7 %) of the patients. In 11 of these patients the pain appears to be associated with biopsy/surgical procedures.
The following adverse reactions have been reported in ≤ 0.5% of patients: signs and symptoms consistent with seizure occurring shortly after administration of the agent; transient arthritis; angioedema, arrhythmia, dizziness, syncope, abdominal pain, vomiting, and severe hypersensitivity characterized by dyspnea, hypotension, bradycardia, asthenia, and vomiting within two hours after a second injection of Technetium Tc 99m Sestamibi. A few cases of flushing, edema, injection site inflammation, dry mouth, fever, pruritis, rash, urticaria and fatigue have also been attributed to administration of the agent.
6DRUG INTERACTIONS
Specific drug-drug interactions have not been studied.
7OVERDOSAGE
The clinical consequences of overdosing with Technetium Tc 99m Sestamibi are not known.
8DESCRIPTION
Each 10 mL vial contains a sterile, non-pyrogenic, lyophilized mixture of:
- Tetrakis (2-methoxy isobutyl isonitrile) Copper (I) tetrafluoroborate - 1 mg
- Sodium Citrate Dihydrate - 2.6 mg
- L-Cysteine Hydrochloride Monohydrate - 1 mg
- Mannitol - 20 mg
- Stannous Chloride, Dihydrate, minimum (SnCl
- Stannous Chloride, Dihydrate (SnCl
- Tin Chloride (stannous and stannic) Dihydrate, maximum (as SnCl
Prior to lyophilization the pH is 5.6 to 5.8. The contents of the vial are lyophilized and stored under nitrogen.
This drug is administered by intravenous injection for diagnostic use after reconstitution with sterile, non-pyrogenic, oxidant-free Sodium Pertechnetate Tc 99m Injection. The pH of the reconstituted product is 5.5 (5.0-6.0). No bacteriostatic preservative is present.
The precise structure of the technetium complex is Tc 99m[MIBI]
8.1Physical Characteristics
Technetium Tc 99m decays by isomeric transition with a physical half-life of 6.02 hours.
1Kocher, David, C., Radioactive Decay Data Tables, DOE/TIC-11026, 108(1981).
8.2External Radiation
The specific gamma ray constant for Tc 99m is 5.4 microcoulombs/kg-MBq-hr (0.78 R/mCi-hr) at 1 cm. The first half value layer is 0.017 cm of Pb. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in
9CLINICAL STUDIES
Clinical Trials:
Myocardial Imaging: In a trial of rest and stress Tc 99m Sestamibi imaging, the relationship of normal or abnormal perfusion scans and long term cardiac events was evaluated in 521 patients (511 men, 10 women) with stable chest pain. There were 73.9 % Caucasians, 25.9 % Blacks and 0.2 % Asians. The mean age was 59.6 years (range: 29 to 84 years). All patients had a baseline rest and exercise Tc 99m Sestamibi scan and were followed for 13.2 ± 4.9 months (range: 1 to 24 months). Images were correlated with the occurrence of a cardiac event (cardiac death or non-fatal myocardial infarction). In this trial as summarized in Table 7, 24/521 (4.6 %) had a cardiac event.
(a) Note: Similar findings were found in two studies with patients who had pharmacologic stress Tc 99m Sestamibi imaging.
Although patients with normal images had a lower cardiac event rate than those with abnormal images, in all patients with abnormal images it was not possible to predict which patient would be likely to have further cardiac events; i.e., such individuals were not distinguishable from other patients with abnormal images.
The findings were not evaluated for defect location, disease duration, specific vessel involvement or intervening management.
In earlier trials, using a template consisting of the anterior wall, inferior-posterior wall and isolated apex, localization in the anterior or inferior-posterior wall in patients with suspected angina or coronary artery disease was shown. Disease localization isolated to the apex has not been established. In adults, Tc 99m Sestamibi has not been studied or evaluated in cardiac disorders other than coronary artery disease.
Breast Imaging: Technetium Tc 99m Sestamibi was evaluated in two multicenter, clinical trials of a total of 673 woman patients. Overall the mean age was 52 (range 23 to 87 years). The racial and ethnic representation was 70% Caucasian, 15 % African-American, 14 % Hispanic and 1% Asian.
Both clinical studies evaluated women who were referred for further evaluation for either: 1) a mammographically detected (with varying degrees of malignant likelihood) but not palpable breast lesion (study A, n=387, mean age = 54 years), or 2) a palpable breast lesion (study B, n=286, mean age = 50 years). In both studies all patients were scheduled for biopsy.
Technetium Tc 99m Sestamibi (20 to 30 mCi) was injected intravenously in a vein that was contralateral to the breast lesion in question. Planar imaging was completed with a high resolution collimator with a 10 % window centered at 140 keV, and 128 x 128 matrix. An initial marker image, that was not used in the data analysis, was obtained using a cobalt Co57 point source as a marker of a palpable mass. Images were obtained 5 minutes after injection as follows: lateral image of the affected breast for 10 minutes, lateral image of the contralateral breast for 10 minutes, and an anterior image of both breasts for 10 minutes. For the lateral image the patients were positioned in a prone position. For the anterior image, the patients were supine. The Technetium Tc 99m Sestamibi scintigraphic images were read in a randomized method by two groups of three blinded readers. Technetium Tc 99m Sestamibi uptake was scored as: normal (no uptake), equivocal, low, moderate, or high uptake. The results of Technetium Tc 99m Sestamibi images and mammography were analyzed in comparison to histopathologic findings of malignant or non-malignant disease.
As shown in
In separate retrospective subset analyses of 259 patients with dense (heterogeneously/extremely dense) and 275 patients with fatty (almost entirely fat/numerous vague densities) breast tissue, the Technetium Tc 99m Sestamibi results were similar. Overall, the studies were not designed to compare the performance of Technetium Tc 99m Sestamibi with the performance of mammography in patients with breast densities or other coexistent breast tissue disorders
In general the histology seems to correlate with the degree of Technetium Tc 99m Sestamibi uptake. As shown in
An estimate of the likelihood of malignancy based on the Technetium Tc 99m Sestamibi uptake score in combination with the mammographic score has not been studied.
In these two studies approximately 150 additional, non-biopsied lesions were found to be positive after Technetium Tc 99m Sestamibi imaging. These lesions were identified in sites that did not physically correlate with identified entry criteria mammographic lesions and these lesions were not palpable. These lesions were not biopsied. Whether these lesions were benign or malignant is not known. Technetium Tc 99m Sestamibi uptake can occur in both benign and malignant disease. THE CLINICAL USEFULNESS OF A POSITIVE Technetium Tc 99m Sestamibi IMAGE IN THE ABSENCE OF AN ABNORMAL MAMMOGRAM OR A PALPABLE LESION IS NOT KNOWN
10HOW SUPPLIED/STORAGE AND HANDLING
Kit for the Preparation of Technetium Tc 99m Sestamibi Injection is supplied as a 10 mL vial in kits of five (5) (NDC # 45548-141-05), ten (10) (NDC # 45548-141-10) and thirty (30) (NDC # 45548-141-30), sterile and non pyrogenic.
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to patient administration. Radiochemical purity should be checked prior to patient administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Prior to lyophilization the pH is between 5.6 and 5.8. The contents of the vial are lyophilized and stored under nitrogen. Store at 15 °C to 25 °C (59 °F to 77 °F) before and after reconstitution.
Kit for Preparation of Technetium Tc 99m Sestamibi Injection contains no preservatives. Included in each kit of five (5) vials, ten (10) vials and thirty (30) vials, is a package insert and a sufficient number of vial shield labels and radiation warning labels.
This reagent kit is approved for distribution to persons licensed by the U.S. Nuclear Regulatory Commission to use by product material identified in 10 CFR 35.200 or under an equivalent license issued by an Agreement State.
11PATIENT COUNSELING INFORMATION
Kit for the Preparation of Technetium Tc 99m Sestamibi Injection contains the same active ingredient as CARDIOLITE
Lactation: Interruption of breastfeeding after exposure to Technetium Tc 99m Sestamibi is not necessary, however, a lactating woman should be advised to consider restricting close contact with her breast fed infant to a maximum of 5 hours in the 24 hour period after Technetium Tc 99m Sestamibi administration in order to minimize radiation exposure [see Use in Specific Populations (.
CARDIOLITE
12PRINCIPAL DISPLAY PANEL - Vial Label
Kit for the preparation of Technetium
Contains 1mg of Tetrakis(2-methoxyisobutylisonitrile)
Diagnostic Agent for Intravenous Use After Labeling with
Store at 15 °C to 25 °C (59 °F to 77 °F)
See package insert for full prescribing
Made in Canada
Manufactured for:
412020
Rx only
13PRINCIPAL DISPLAY PANEL - 10 Vials Carton Label
Kit for the Preparation of
Diagnostic Agent for Intravenous Use After Labeling
500141
Manufactured for:
Made in Canada
Rx onlySee Package Insert for full prescribing Information.
WARNING: Radiopharmaceuticals should be used by persons who are qualified by specific training in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
CONTENTS AND STORAGE CONDITIONS:10 Vials each containing sterile and non-pyrogenic:
1mg of Tetrakis(2-methoxyisobutylisonitrile)copper (I) Tetrafluoroborate, 0.075mg of Stannous Chloride Dihydrate, 1mg of L-Cysteine Hydrochloride Monohydrate, 2.6mg of Sodium Citrate Dihydrate and 20mg of Mannitol.
10 Radioactive Material Caution labels
10 Radioassay information labels with radiation symbol
1 Package Insert
Store at 15 °C to 25°C (59 °F to 77°F) See Package Insert for dosage information. Reconstitute with additive-free Tc 99m and store at 15° to 25°C (59° to 77°F). Use within 6 hours of reconstitution.
WARNING: Radiopharmaceuticals should be used by persons who are qualified by specific training in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
CONTENTS AND STORAGE CONDITIONS:10 Vials each containing sterile and non-pyrogenic:
1mg of Tetrakis(2-methoxyisobutylisonitrile)copper (I) Tetrafluoroborate, 0.075mg of Stannous Chloride Dihydrate, 1mg of L-Cysteine Hydrochloride Monohydrate, 2.6mg of Sodium Citrate Dihydrate and 20mg of Mannitol.
10 Radioactive Material Caution labels
10 Radioassay information labels with radiation symbol
1 Package Insert
Store at 15 °C to 25°C (59 °F to 77°F) See Package Insert for dosage information. Reconstitute with additive-free Tc 99m and store at 15° to 25°C (59° to 77°F). Use within 6 hours of reconstitution.
14PRINCIPAL DISPLAY PANEL - Caution Label For Radioactive Material
CAUTION
15PRINCIPAL DISPLAY PANEL - RADIOASSAY INFORMATION LABEL
CAUTION
RADIOACTIVE
MATERIAL
Rx Only
Technetium Tc 99m
Sestamibi Injection
Contents
Sodium Pertechnetate Tc 99m Injection
Tetrakis (2-methoxyisobutylisonitrile)
copper(I) tetrafluoroborate - 1mg
Stannous Chloride
Dihydrate - 0.075mg
L-Cysteine Hydrochloride
Monohydrate - 1mg
Sodium Citrate Dihydrate - 2.6mg
Mannitol - 20mg
Store at 15o to 25oC (59o to 77oF).
Use within 6 hours of reconstitution.
216511
MBq (mCi) Tc 99m/mL
Volume.................mL
Time/Date.................
Expiration Time.........
Lot No......................
Technetium Tc 99m
Sestamibi Injection
216511