Levemir
What is Levemir (Detemir)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This study compares insulin icodec, taken once a week, with other basal insulins, taken once a day, in people with type 2 diabetes.The purpose of this study is to see how well the approved injectable weekly insulin icodec maintains blood sugar levels when compared to approved and available daily injectable basal insulins in people with type 2 diabetes. The participants will either be prescribed we...
Summary: This study will look into testing a new medicine called NNC0363-1063 which may be used to treat people with diabetes. The study consists of three parts: Part 1 is a single ascending dose (SAD) study that comprises two subtypes: Part 1A conducted in healthy participants and Part 1B conducted in participants with type 1 diabetes (T1D). This study part will last for about 1½ to 5½ weeks. Part 2 is a ...
Summary: This randomized controlled clinical trial will assess whether continuation of home oral antidiabetic agents during hospitalization can be used as a safe and effective alternative to insulin therapy in the management of diabetes in the hospital. The primary outcome of the study is to determine differences in glycemic control as measured by mean daily blood glucose concentration between oral antidia...
Related Latest Advances
Brand Information
- 3 mL single-patient-use FlexPen prefilled pen
- 10 mL multiple-dose vial
- During episodes of hypoglycemia
- In patients with hypersensitivity to insulin detemir or any of the excipients in LEVEMIR. Reactions have included anaphylaxis
- Hypoglycemia
- Hypoglycemia Due to Medication errors
- Hypersensitivity Reactions
- Hypokalemia
- Hypersensitivity Reactions
- Severe, life-threatening, generalized allergy, including anaphylaxis, generalized skin reactions, angioedema, bronchospasm, hypotension, and shock have occurred with insulin, including LEVEMIR, and may be life-threatening.
- Do not share your Levemir FlexTouch Pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
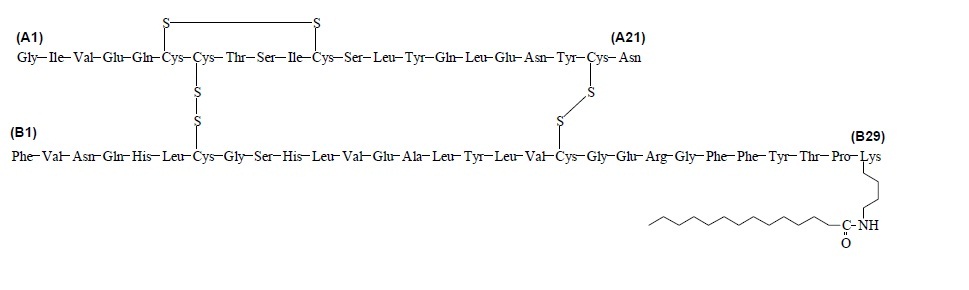
- Levemir FlexTouch Pen (“Pen”) is a prefilled disposable, single-patient-use insulin pen containing 300 units insulin detemir. You can inject from 1 to 80 units in a single injection.
- People who are blind or have vision problems should not use this Pen without help from a person trained to use the Pen.
- Levemir FlexTouch Pen
- a new NovoFine, NovoFine Plus or NovoTwist needle
- alcohol swab
- 1 sharps container for throwing away used Pens and needles.
- Wash your hands with soap and water.
- Before you start to prepare your injection, check the Levemir FlexTouch Pen label to make sure you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin.
- Levemir should look clear and colorless.
- Do not use Levemir past the expiration date printed on the label or 42 days after you start using the Pen.
- Always use a new needle for each injection to help ensure sterility and prevent blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection, or get a serious infection from them.
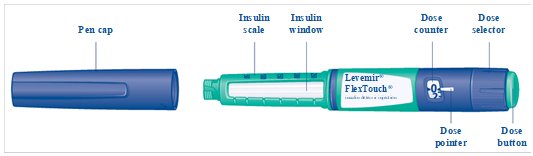
- Inject your Levemir exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- Levemir can be injected under the skin (subcutaneously) of your stomach area (abdomen), upper legs (thighs) or upper arms.
- For each injection, change (rotate) your injection site within the area of skin that you use to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- The used Levemir FlexTouch Pen may be thrown away in your household trash after you have removed the needle.
- You can put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Store unused Levemir FlexTouch Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Store the Pen you are currently using out of the refrigerator up to 86°F.
- Do not freeze Levemir. Do not use Levemir if it has been frozen.
- Keep Levemir away from heat or light.
- Unused Pens may be used until the expiration date printed on the label, if kept in the refrigerator.
- The Levemir FlexTouch Pen you are using should be thrown away after 42 days, even if it still has insulin left in it.
- Keep Levemir FlexTouch Pens and needles out of the reach of children.
- Always use a new needle for each injection.
- Do not share your Levemir FlexTouch Pen or needles with other people. You may give other people a serious infection, or get a serious infection from them.
Novo Nordisk Inc.

 People who are blind or have vision problems should not use this Pen without help from a person trained to use the Pen.
People who are blind or have vision problems should not use this Pen without help from a person trained to use the Pen.- LEVEMIR FlexPen
- NovoFine or NovoFine Plus disposable needles
- Alcohol swab
- Sharps disposal container (see
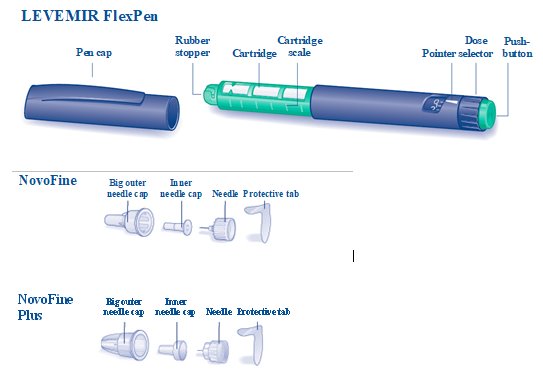
 Always use a new needle for each injection to make sure the needle is free of germs (sterile) and to prevent blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection or get a serious infection from them.
Always use a new needle for each injection to make sure the needle is free of germs (sterile) and to prevent blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection or get a serious infection from them. Be careful not to bend or damage the needle before use.
Be careful not to bend or damage the needle before use. To reduce the risk of needle sticks, never put the inner needle cap back on the needle.
To reduce the risk of needle sticks, never put the inner needle cap back on the needle. - If you do not have a sharps container, carefully slip the needle into the outer needle cap using 1 hand. Use your other hand to pinch the base of the big outer needle cap and unscrew the used needle from the Pen and throw it away as soon as you can.
- The used LEVEMIR FlexPen may be thrown away in your household trash after you have removed the needle.
- Put your used needles in an FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
- Store the unused (unopened) LEVEMIR FlexPen in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Store the LEVEMIR FlexPen you are currently using out of the refrigerator up to 86°F.
- Do not freeze the LEVEMIR FlexPen. Do not use the LEVEMIR FlexPen if it has been frozen.
- Keep the LEVEMIR FlexPen away from heat or light.
- Unused LEVEMIR FlexPens may be used until the expiration date printed on the label, if kept in the refrigerator.
 Remove the needle from the LEVEMIR FlexPen after each injection. This helps to ensure sterility, prevent leakage of insulin, and will help to make sure you inject the right dose of insulin for future injections.
Remove the needle from the LEVEMIR FlexPen after each injection. This helps to ensure sterility, prevent leakage of insulin, and will help to make sure you inject the right dose of insulin for future injections. Be careful when handling used needles to avoid needle sticks and transfer of infectious diseases.
Be careful when handling used needles to avoid needle sticks and transfer of infectious diseases. Use the LEVEMIR FlexPen exactly as your healthcare provider tells you to.
Use the LEVEMIR FlexPen exactly as your healthcare provider tells you to. Do not share your LEVEMIR FlexPen or needles with other people. You may give other people a serious infection, or get a serious infection from them.
Do not share your LEVEMIR FlexPen or needles with other people. You may give other people a serious infection, or get a serious infection from them. Always use a new needle for each injection.
Always use a new needle for each injection. Novo Nordisk is not responsible for harm due to using this insulin pen with products not recommended by Novo Nordisk.
Novo Nordisk is not responsible for harm due to using this insulin pen with products not recommended by Novo Nordisk. As a precautionary measure, always carry a spare insulin delivery device in case your LEVEMIR FlexPen is lost or damaged.
As a precautionary measure, always carry a spare insulin delivery device in case your LEVEMIR FlexPen is lost or damaged.  Remember to keep the disposable LEVEMIR FlexPen with you. Do not leave it in a car or other location where it can get too hot or too cold.
Remember to keep the disposable LEVEMIR FlexPen with you. Do not leave it in a car or other location where it can get too hot or too cold.



