Brand Name
Mesnex
Generic Name
Mesna
View Brand Information FDA approval date: December 30, 1988
Classification: Cytoprotective Agent
Form: Injection, Tablet, Solution
What is Mesnex (Mesna)?
Mesna Injection is indicated as a prophylactic agent in reducing the incidence of ifosfamide-induced hemorrhagic cystitis. Limitation of Use: Mesna Injection is not indicated to reduce the risk of hematuria due to other pathological conditions such as thrombocytopenia. Mesna Injection is a cytoprotective agent indicated as a prophylactic agent in reducing the incidence of ifosfamide-induced hemorrhagic cystitis. Limitation of Use: Mesna Injection is not indicated to reduce the risk of hematuria due to other pathological conditions such as thrombocytopenia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
MESNEX (mesna)
1INDICATIONS AND USAGE
MESNEX is indicated as a prophylactic agent in reducing the incidence of ifosfamide-induced hemorrhagic cystitis.
Limitation of Use:
MESNEX is not indicated to reduce the risk of hematuria due to other pathological conditions such as thrombocytopenia.
MESNEX is not indicated to reduce the risk of hematuria due to other pathological conditions such as thrombocytopenia.
2DOSAGE FORMS AND STRENGTHS
- MESNEX (mesna) injection: 1 g Multidose Vial, 100 mg/mL
- MESNEX (mesna) tablets: 400 mg film-coated tablets with functional score
3CONTRAINDICATIONS
MESNEX is contraindicated in patients known to be hypersensitive to mesna or to any of the excipients [see
4ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling.
- Hypersensitivity Reactions
- Dermatological Toxicity
- Benzyl Alcohol Toxicity
- Laboratory Test Interferences
- Use in Patients with a History of Adverse Reactions to Thiol Compounds
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
MESNEX adverse reaction data are available from four Phase 1 studies in which single intravenous doses of 600-1200 mg MESNEX injection without concurrent chemotherapy were administered to a total of 53 healthy volunteers and single oral doses of 600-2400 mg of MESNEX tablets were administered to a total of 82 healthy volunteers. The most frequently reported side effects (observed in two or more healthy volunteers) for healthy volunteers receiving single doses of MESNEX injection alone were headache, injection site reactions, flushing, dizziness, nausea, vomiting, somnolence, diarrhea, anorexia, fever, pharyngitis, hyperesthesia, influenza-like symptoms, and coughing. In two Phase 1 multiple-dose studies where healthy volunteers received MESNEX tablets alone or intravenous MESNEX followed by repeated doses of MESNEX tablets, flatulence and rhinitis were reported. In addition, constipation was reported by healthy volunteers who had received repeated doses of intravenous MESNEX.
Additional adverse reactions in healthy volunteers receiving MESNEX alone included injection site reactions, abdominal pain/colic, epigastric pain/burning, mucosal irritation, lightheadedness, back pain, arthralgia, myalgia, conjunctivitis, nasal congestion, rigors, paresthesia, photophobia, fatigue, lymphadenopathy, extremity pain, malaise, chest pain, dysuria, pleuritic pain, dry mouth, dyspnea, and hyperhidrosis. In healthy volunteers, MESNEX was commonly associated with a rapid (within 24 hours) decrease in lymphocyte count, which was generally reversible within one week of administration.
Because MESNEX is used in combination with ifosfamide or ifosfamide-containing chemotherapy regimens, it is difficult to distinguish the adverse reactions which may be due to MESNEX from those caused by the concomitantly administered cytotoxic agents.
Adverse reactions reasonably associated with MESNEX administered intravenously and orally in four controlled studies in which patients received ifosfamide or ifosfamide-containing regimens are presented in Table 3.
4.2Postmarketing Experience
The following adverse reactions have been reported in the postmarketing experience of patients receiving MESNEX in combination with ifosfamide or similar drugs, making it difficult to distinguish the adverse reactions which may be due to MESNEX from those caused by the concomitantly administered cytotoxic agents. Because these reactions are reported from a population of unknown size, precise estimates of frequency cannot be made.
Cardiovascular: Hypertension
Gastrointestinal: Dysgeusia
Hepatobiliary: Hepatitis
Nervous System: Convulsion
Respiratory: Hemoptysis
5DRUG INTERACTIONS
No clinical drug interaction studies have been conducted with MESNEX.
6OVERDOSAGE
There is no known antidote for MESNEX.
In a clinical trial, 11 patients received intravenous MESNEX 10 mg/kg to 66 mg/kg per day for 3 to 5 days. Patients also received ifosfamide or cyclophosphamide. Adverse reactions included nausea, vomiting, diarrhea and fever. An increased rate of these adverse reactions has also been found in oxazaphosphorine-treated patients receiving ≥80 mg MESNEX per kg per day intravenously compared with patients receiving lower doses or hydration treatment only.
Postmarketing, administration of 4.5 g to 6.9 g of MESNEX resulted in hypersensitivity reactions including mild hypotension, shortness of breath, asthma exacerbation, rash, and flushing.
7DESCRIPTION
MESNEX (mesna) is a detoxifying agent to inhibit the hemorrhagic cystitis induced by ifosfamide. The active ingredient, mesna, is a synthetic sulfhydryl compound designated as sodium-2-mercaptoethane sulfonate with a molecular formula of C
HS–CH
MESNEX injection is a sterile, nonpyrogenic, aqueous solution of clear and colorless appearance in clear glass multidose vials for intravenous administration. MESNEX injection contains 100 mg/mL mesna, 0.25 mg/mL edetate disodium and sodium hydroxide for pH adjustment. MESNEX injection multidose vials also contain 10.4 mg/mL of benzyl alcohol as a preservative. The solution has a pH range of 7.5-8.5.
MESNEX tablets are white, oblong, scored biconvex film-coated tablets with the imprint M4. They contain 400 mg mesna. The excipients are calcium phosphate, cornstarch, hydroxypropylmethylcellulose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, simethicone, and titanium dioxide.
8HOW SUPPLIED/STORAGE AND HANDLING
MESNEX (mesna) injection 100 mg/mL
- NDC 0338-1305-01 1 g Multidose Vial, Box of 1 vial of 10 mL
- NDC 0338-1305-03 1 g Multidose Vial, Box of 10 vials of 10 mL
- Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]
If MESNEX is co-administered with ifosfamide, refer to the ifosfamide prescribing information for safe handling instructions.
MESNEX (mesna) tablets
- NDC 67108-3565-9 400 mg scored tablets packaged in box of 10 tablets
- Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]
9PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Hypersensitivity
- Advise the patient to discontinue MESNEX and seek immediate medical attention if any signs or symptoms of a hypersensitivity reaction, including systemic anaphylactic reactions occur
Dosing Instructions
- Advise the patient to take MESNEX at the exact time and in the exact amount as prescribed. Advise the patient to contact their healthcare provider if they vomit within 2 hours of taking oral MESNEX, or if they miss a dose of oral MESNEX
Hemorrhagic Cystitis
- MESNEX does not prevent hemorrhagic cystitis in all patients nor does it prevent or alleviate any of the other adverse reactions or toxicities associated with ifosfamide. Advise the patient to report to their healthcare provider if his/her urine has turned a pink or red color
- Advise the patient to drink 1 to 2 liters of fluid each day during MESNEX therapy
Dermatologic Toxicity
- Advise the patient that Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug rash with eosinophilia and systemic symptoms and bullous and ulcerative skin and mucosal reactions have occurred with MESNEX. Advise the patient to report to their healthcare provider if signs and symptoms of these syndromes occur
Benzyl Alcohol Toxicity
- Advise patients that serious adverse reactions are associated with the benzyl alcohol found in MESNEX and other medications in premature neonates and low-birth weight infants
Embryo-Fetal Toxicity
- MESNEX is used in combination with ifosfamide. Ifosfamide or other cytotoxic agents can cause fetal harm when administered to a pregnant woman. Inform female patients of the risk to a fetus and potential loss of the pregnancy. Advise females to inform their healthcare provider if they are pregnant or become pregnant
Contraception
- Advise females of reproductive potential to use effective contraception during treatment with MESNEX in combination with ifosamide and for 6 months after the last dose
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment with MESNEX in combination with ifosamide and for 3 months after the last dose
Lactation
- Advise lactating women not to breastfeed during treatment with MESNEX or ifosfamide and for 1 week after the last dose
10PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
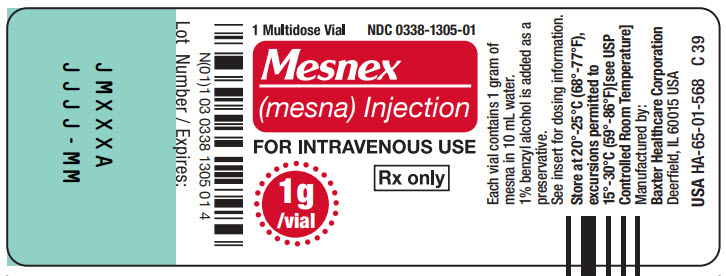
1g Multidose Vial
1 Multidose Vial NDC 0338-1305-01
Mesnex
FOR INTRAVENOUS USE
1g/vial Rx only
N (01) 1 03 0338 1305 01 4
Lot Number / Expires:
JMXXXA
JJJJ - MM
Each vial contains 1 gram of
Manufactured by:
USAHA-65-01-568 C 93



1 Multidose Vial
MESNEX
1g/vial
FOR INTRAVENOUS USE
Rx only
BaxterLogo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
(01) 00303381305017
Barcode
MESNEX
1g/vial
Barcode
HA-80-02-158
Fragile:
BaxterLogo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
C
1 Multidose Vial
MESNEX
1g/vial
FOR INTRAVENOUS USE
Rx only
BaxterLogo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Lot/Exp:
2639B3994
Barcode
Folding Box Logo
Each vial contains 1 g mesna in
Dosage: See package insert for
Manufactured by:
Made in Germany

Schwarz
P 186 C
10 Multidose Vials NDC 0338-1305-03
1g
MESNEX
(mesna) Injection
BaxterLogo
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Manufactured by
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
10 Multidose Vials NDC 0338-1305-03
1g
N3 0338130503 1
MESNEX
Each vial contains 1 g mesna in 10mL water. Mesnex is a sterile and nonpyrogenic solution
Fragile:Handle with care. Store at 20° - 25°C (68°-77°F) see excursions permitted to 15° - 30°C
(59° - 86°F) [see USP Controlled Room Temperature] U.S. Patent No 5,696, 172
(59° - 86°F) [see USP Controlled Room Temperature] U.S. Patent No 5,696, 172
FOR INTRAVENOUS USE
Dosage:See package insert for directions for use. Should not be prescribed without thorough
knowledge of dose, indications and toxicology as contained in accompanying literature.
knowledge of dose, indications and toxicology as contained in accompanying literature.
Manufactured by:
Made in Germany
Rx only
HA-80-01-627
10 Multidose Vials NDC 0338-1305-03
1g
MESNEX
FOR INTRAVENOUS USE
10 Multidose Vials
10 Multidose Vials
C
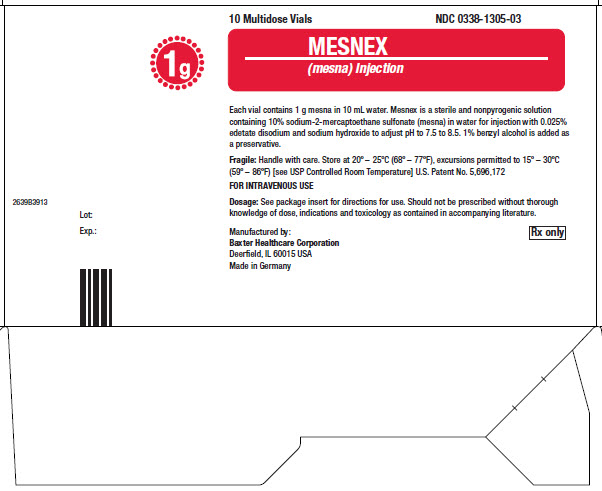
10 Multidose Vials NDC 0338-1305-03
1g
2639B3913
Lot:
MESNEX
Each vial contains 1 g mesna in 10mL water. Mesnex is a sterile and nonpyrogenic solution
Fragile:Handle with care. Store at 20° - 25°C (68°-77°F) see excursions permitted to 15° - 30°C
(59° - 86°F) [see USP Controlled Room Temperature] U.S. Patent No 5,696, 172
(59° - 86°F) [see USP Controlled Room Temperature] U.S. Patent No 5,696, 172
FOR INTRAVENOUS USE
Dosage:See package insert for directions for use. Should not be prescribed without thorough
knowledge of dose, indications and toxicology as contained in accompanying literature.
knowledge of dose, indications and toxicology as contained in accompanying literature.
Manufactured by:
Made in Germany
Rx only
Folding Box
10 Multidose Vials NDC 0338-1305-03
1g
MESNEX
FOR INTRAVENOUS USE
10 Multidose Vials
10 Multidose Vials