Ninlaro
What is Ninlaro (Ixazomib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This study will independently assess the efficacy and safety of 11 combination therapies in 12 arms, in dose-escalation/-evaluation and expansion phases, for the treatment of patients with relapsed/refractory multiple myeloma (RRMM) and newly diagnosed multiple myeloma (NDMM). The combinations to be evaluated are: * Arm 1: Selinexor + dexamethasone + pomalidomide (SPd); enrollment complete * Arm 2...
Summary: The main aim of this study is to check side effects and results in adults with multiple myeloma after switching from a bortezomib/carfilzomib -based to an Ixazomib-based treatment.
Summary: The study seeks to investigate safety and efficacy of ixazomib (NINLARO), a proteasome inhibitor, in multiple sclerosis (MS). Participants will receive either ixazomib capsules or placebo capsules for up to 24 months.
Related Latest Advances
Brand Information
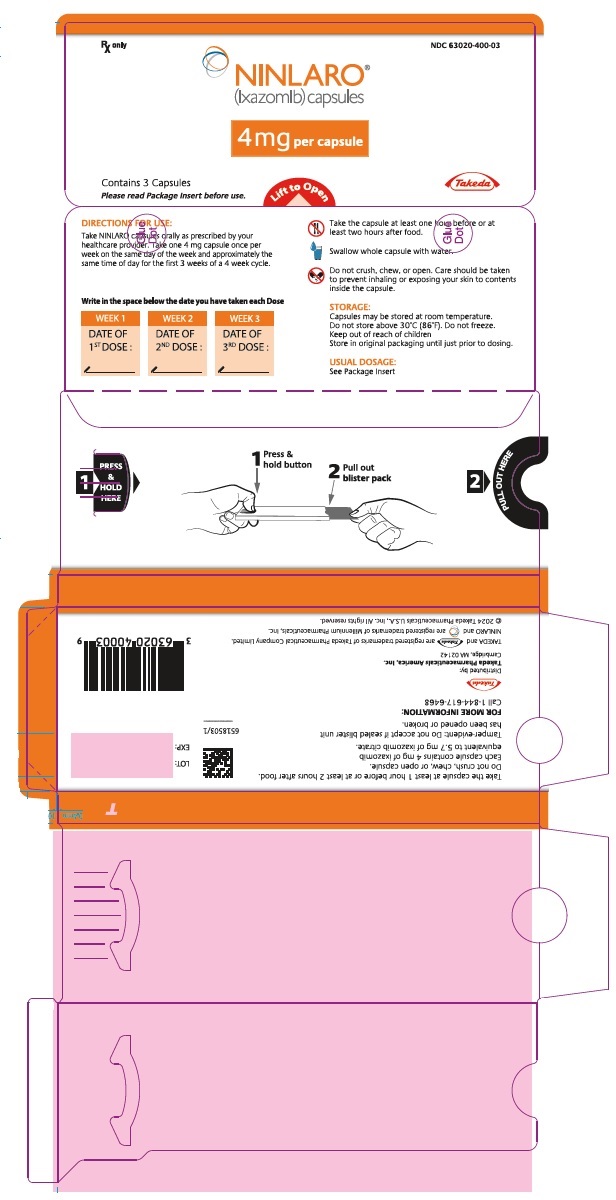
- 4 mg ixazomib: Light orange gelatin capsule imprinted with "Takeda" on the cap and "4 mg" on the body in black ink.
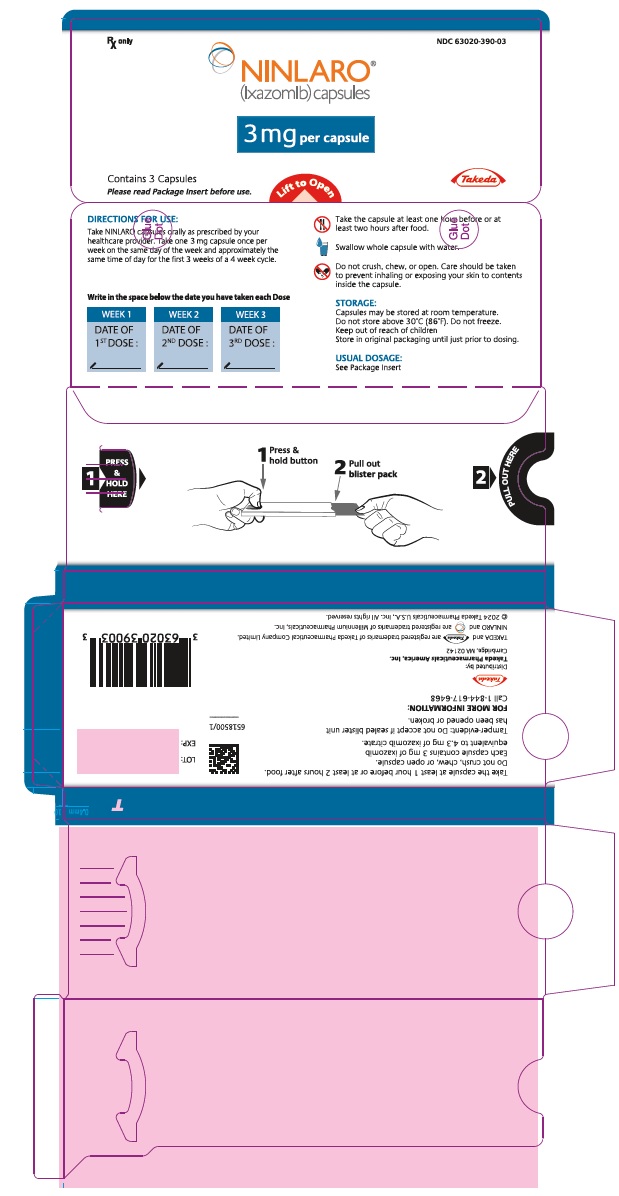
- 3 mg ixazomib: Light grey gelatin capsule imprinted with "Takeda" on the cap and "3 mg" on the body in black ink.
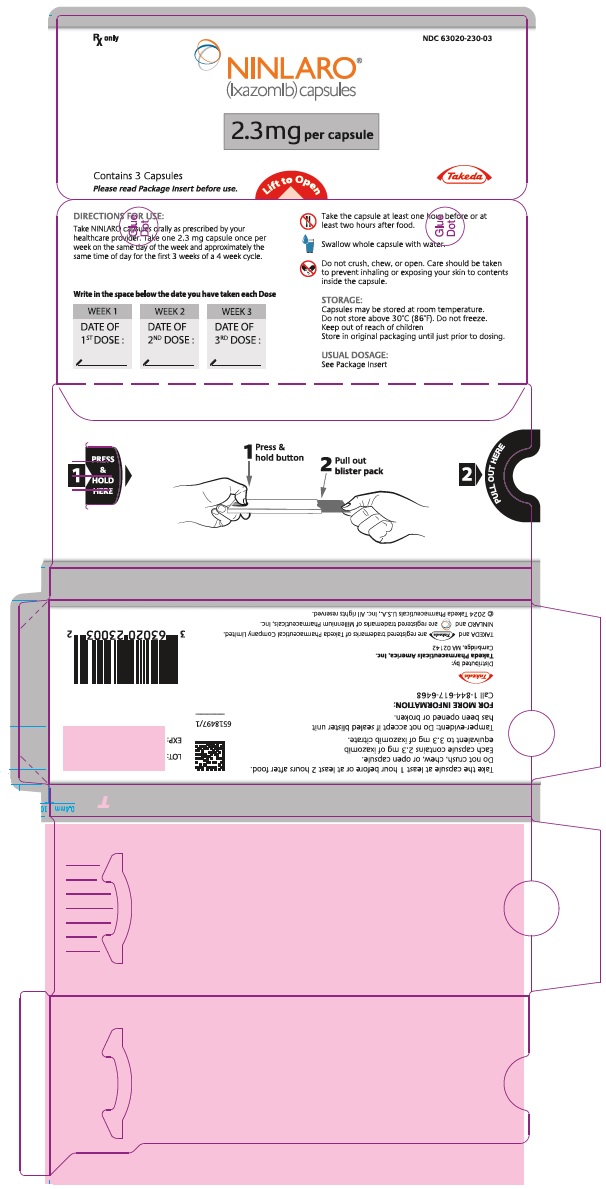
- 2.3 mg ixazomib: Light pink gelatin capsule imprinted with "Takeda" on the cap and "2.3 mg" on the body in black ink.
- Thrombocytopenia
- Gastrointestinal Toxicities
- Peripheral Neuropathy
- Peripheral Edema
- Cutaneous Reactions
- Thrombotic Microangiopathy
- Hepatotoxicity
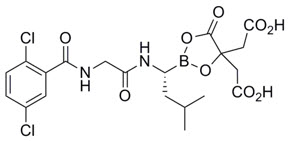
- OSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
(ixazomib) capsules
(ixazomib) capsules
(ixazomib) capsules