Brand Name
Survanta
Generic Name
Beractant
View Brand Information FDA approval date: July 01, 1991
Form: Suspension
What is Survanta (Beractant)?
SURVANTA is indicated for prevention and treatment of Respiratory Distress Syndrome in premature infants.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Survanta (Beractant)
1DESCRIPTION
SURVANTA
SURVANTA (beractant) intratracheal suspension is a sterile, preservative-free, non-pyrogenic off-white to light brown liquid supplied in single-dose glass vials for intratracheal use only. Each vial contains 4 mL (100 mg phospholipids) or 8 mL (200 mg phospholipids). Each mL of SURVANTA contains 25 mg of phospholipids. It is suspended in 0.9% sodium chloride solution and heat-sterilized. SURVANTA contains no preservatives. Sodium hydroxide or hydrochloric acid may be added to adjust the pH. The pH is approximately 6.2 to 7.6.
2CLINICAL PHARMACOLOGY
Endogenous pulmonary surfactant lowers surface tension on alveolar surfaces during respiration and stabilizes the alveoli against collapse at resting transpulmonary pressures. Deficiency of pulmonary surfactant causes Respiratory Distress Syndrome (RDS) in premature infants. SURVANTA replenishes surfactant and restores surface activity to the lungs of these infants.
2.1Activity
In vitro, SURVANTA reproducibly lowers minimum surface tension to less than 8 dynes/cm as measured by the pulsating bubble surfactometer and Wilhelmy Surface Balance. In situ, SURVANTA restores pulmonary compliance to excised rat lungs artificially made surfactant-deficient. In vivo, single SURVANTA doses improve lung pressure-volume measurements, lung compliance, and oxygenation in premature rabbits and sheep.
2.2Animal Metabolism
SURVANTA is administered directly to the target organ, the lungs, where biophysical effects occur at the alveolar surface. In surfactant-deficient premature rabbits and lambs, alveolar clearance of radio-labelled lipid components of SURVANTA is rapid. Most of the dose becomes lung-associated within hours of administration, and the lipids enter endogenous surfactant pathways of reutilization and recycling. In surfactant-sufficient adult animals, SURVANTA clearance is more rapid than in premature and young animals. There is less reutilization and recycling of surfactant in adult animals.
Limited animal experiments have not found effects of SURVANTA on endogenous surfactant metabolism. Precursor incorporation and subsequent secretion of saturated phosphatidylcholine in premature sheep are not changed by SURVANTA treatments.
No information is available about the metabolic fate of the surfactant-associated proteins in SURVANTA. The metabolic disposition in humans has not been studied.
3CLINICAL STUDIES
Clinical effects of SURVANTA were demonstrated in six single-dose and four multiple-dose randomized, multi-center, controlled clinical trials involving approximately 1700 infants. Three open trials, including a Treatment IND, involved more than 8500 infants. Each dose of SURVANTA in all studies was 100 mg phospholipids/kg birth weight and was based on published experience with Surfactant TA, a lyophilized powder dosage form of SURVANTA having the same composition. SURVANTA significantly reduces the incidence of RDS, mortality due to RDS and air leak complications.
3.1Prevention Studies
Infants of 600-1250 g birth weight and 23 to 29 weeks estimated gestational age were enrolled in two multiple-dose studies. A dose of SURVANTA was given within 15 minutes of birth to prevent the development of RDS. Up to three additional doses in the first 48 hours, as often as every 6 hours, were given if RDS subsequently developed and infants required mechanical ventilation with an FiO
3.2Rescue Studies
Infants of 600-1750 g birth weight with RDS requiring mechanical ventilation and an FiO
3.3Acute Clinical Effects
Marked improvements in oxygenation may occur within minutes of administration of SURVANTA.
All controlled clinical studies with SURVANTA provided information regarding the acute effects of SURVANTA on the arterial-alveolar oxygen ratio (a/APO
4INDICATIONS AND USAGE
SURVANTA is indicated for prevention and treatment (“rescue”) of Respiratory Distress Syndrome (RDS) (hyaline membrane disease) in premature infants.
5CONTRAINDICATIONS
None
6WARNINGS
SURVANTA can rapidly affect oxygenation and lung compliance within minutes of administration of SURVANTA. Therefore, its use should be restricted to a highly supervised clinical setting with immediate availability of clinicians experienced with intubation, ventilator management, and general care of premature infants. Infants receiving SURVANTA should be frequently monitored with arterial or transcutaneous measurement of systemic oxygen and carbon dioxide.
During the dosing procedure, transient episodes of bradycardia and decreased oxygen saturation have been reported. If these occur, stop the dosing procedure and initiate appropriate measures to alleviate the condition. After stabilization, resume the dosing procedure.
7ADVERSE REACTIONS
The most commonly reported adverse experiences were associated with the dosing procedure. In the multiple-dose controlled clinical trials, each dose of SURVANTA was divided into four quarter-doses which were instilled through a catheter inserted into the endotracheal tube by briefly disconnecting the endotracheal tube from the ventilator. Transient bradycardia occurred with 11.9% of
Other reactions during the dosing procedure occurred with fewer than 1% of doses and included endotracheal tube reflux, pallor, vasoconstriction, hypotension, endotracheal tube blockage, hypertension, hypocarbia, hypercarbia, and apnea. No deaths occurred during the dosing procedure, and all reactions resolved with symptomatic treatment.
The occurrence of concurrent illnesses common in premature infants was evaluated in the controlled trials. The rates in all controlled studies are in Table 3.
When all controlled studies were pooled, there was no difference in intracranial hemorrhage. However, in one of the single-dose rescue studies and one of the multiple-dose prevention studies, the rate of intracranial hemorrhage was significantly higher in SURVANTA patients than control patients (63.3%
In the controlled clinical trials, there was no effect of SURVANTA on results of common laboratory tests: white blood cell count and serum sodium, potassium, bilirubin, and creatinine.
More than 4300 pretreatment and post-treatment serum samples from approximately 1500 patients were tested by Western Blot Immunoassay for antibodies to surfactant-associated proteins SP-B and SP-C. No IgG or IgM antibodies were detected.
Several other complications are known to occur in premature infants. The following conditions were reported in the controlled clinical studies. The rates of the complications were not different in treated and control infants, and none of the complications were attributed to SURVANTA.
7.1Respiratory
lung consolidation, blood from the endotracheal tube, deterioration after weaning, respiratory decompensation, subglottic stenosis, paralyzed diaphragm, respiratory failure.
7.2Cardiovascular
hypotension, hypertension, tachycardia, ventricular tachycardia, aortic thrombosis, cardiac failure, cardio-respiratory arrest, increased apical pulse, persistent fetal circulation, air embolism, total anomalous pulmonary venous return.
7.3Gastrointestinal
abdominal distention, hemorrhage, intestinal perforations, volvulus, bowel infarct, feeding intolerance, hepatic failure, stress ulcer.
7.4Renal
renal failure, hematuria.
7.5Hematologic
coagulopathy, thrombocytopenia, disseminated intravascular coagulation.
7.6Central Nervous System
seizures
7.7Endocrine/Metabolic
adrenal hemorrhage, inappropriate ADH secretion, hyperphosphatemia.
7.8Musculoskeletal
inguinal hernia.
7.9Systemic
fever, deterioration.
7.10Follow-Up Evaluations
To date, no long-term complications or sequelae of SURVANTA therapy have been found.
Single-Dose Studies
Six-month adjusted-age follow-up evaluations of 232 infants (115 treated) demonstrated no clinically important differences between treatment groups in pulmonary and neurologic sequelae, incidence or severity of retinopathy of prematurity, rehospitalizations, growth, or allergic manifestations.
Multiple-Dose Studies
Six-month adjusted age follow-up evaluations have been completed in 631 (345 treated) of 916 surviving infants. There were significantly less cerebral palsy and need for supplemental oxygen in SURVANTA infants than controls. Wheezing at the time of examination was significantly more frequent among SURVANTA infants, although there was no difference in bronchodilator therapy.
Final twelve-month follow-up data from the multiple-dose studies are available from 521 (272 treated) of 909 surviving infants. There was significantly less wheezing in SURVANTA infants than controls, in contrast to the six-month results. There was no difference in the incidence of cerebral palsy at twelve months.
Twenty-four month adjusted age evaluations were completed in 429 (226 treated) of 906 surviving infants. There were significantly fewer SURVANTA infants with rhonchi, wheezing, and tachypnea at the time of examination. No other differences were found.
8OVERDOSAGE
Overdosage with SURVANTA has not been reported. Based on animal data, overdosage might result in acute airway obstruction. Treatment should be symptomatic and supportive.
Rales and moist breath sounds can transiently occur after SURVANTA is given, and do not indicate overdosage. Endotracheal suctioning or other remedial action is not required unless clear-cut signs of airway obstruction are present.
9HOW SUPPLIED
SURVANTA (beractant) intratracheal suspension is supplied in 100 mg/4 mL single-dose glass vials (NDC 0074-1040-04) or 200 mg/8 mL single-dose glass vials (NDC 0074-1040-08). Each mL contains 25 mg of phospholipids suspended in 0.9% sodium chloride solution. The color is off-white to light brown.
Store unopened vials refrigerated at 36°F to 46°F (2°C to 8°C). Do not shake. Protect from light. Store vials in carton until ready for use. Vials are for one-time use and for only one patient. Upon opening, discard unused drug.
LITHO IN USA
AbbVie Inc.
North Chicago, IL 60064, U.S.A.
US License Number 1889
20065798 October, 2020
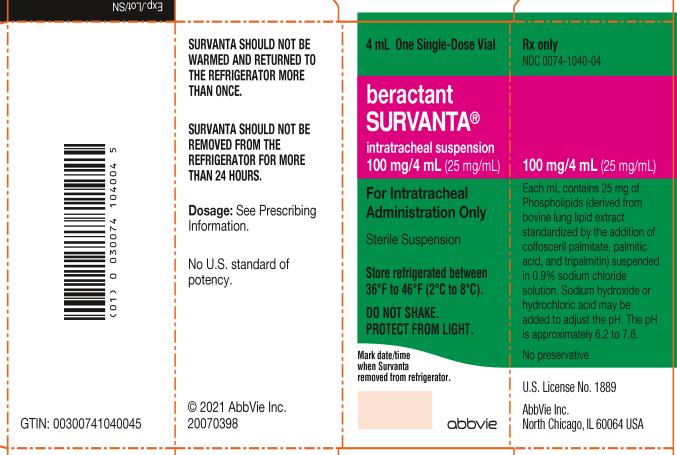
10Principal Display Panel
NDC 0074-1040-08
For Intratracheal
Administration Only
Administration Only
Sterile Suspension
Store refrigerated between
36ºF to 46ºF (2ºC to 8ºC).
36ºF to 46ºF (2ºC to 8ºC).
DO NOT SHAKE.
PROTECT FROM LIGHT.
PROTECT FROM LIGHT.
Rx only
abbvie
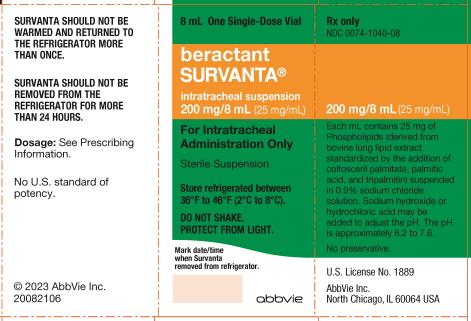
11Principal Display Panel
NDC 0074-1040-04
For Intratracheal
Administration Only
Administration Only
Sterile Suspension
Store refrigerated between
36ºF to 46ºF (2ºC to 8ºC).
36ºF to 46ºF (2ºC to 8ºC).
DO NOT SHAKE.
PROTECT FROM LIGHT.
PROTECT FROM LIGHT.
Rx only
abbvie