Generic Name
Desloratadine
Brand Names
Clarinex-D 12, Clarinex
FDA approval date: January 11, 2013
Classification: Histamine-1 Receptor Antagonist
Form: Tablet
What is Clarinex-D 12 (Desloratadine)?
Desloratadine is a histamine-1 receptor antagonist indicated for: Seasonal Allergic Rhinitis: relief of nasal and non-nasal symptoms in patients 2 years of age and older.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
CLARINEX-D 12 HOUR (desloratadine and pseudoephedrine sulfate)
1DOSAGE AND ADMINISTRATION
Administer CLARINEX-D 12 HOUR Extended Release Tablet by the oral route only. Do not break, chew, or crush the tablet. Swallow the tablet whole.
1.1Adults and Adolescents 12 years of Age and Over
The recommended dose of CLARINEX-D 12 HOUR Extended Release Tablets is 1 tablet twice a day, administered approximately 12 hours apart and with or without a meal. Higher doses or increased dosing frequency of CLARINEX-D 12 HOUR Extended Release Tablets have not demonstrated increased effectiveness. Do not exceed the recommended dose as desloratadine and pseudoephedrine, the active components of CLARINEX-D 12 HOUR Extended Release Tablets have been associated with adverse effects at higher doses
2DOSAGE FORMS AND STRENGTHS
CLARINEX-D 12 HOUR Extended Release Tablets are oval shaped, blue and white bilayer tablets with "D12" embossed in the blue layer. Each tablet contains 2.5 mg desloratadine in the blue immediate-release layer and 120 mg of pseudoephedrine sulfate USP in the white extended-release layer.
3CONTRAINDICATIONS
CLARINEX-D 12 HOUR Extended Release Tablets are contraindicated in:
- Patients with hypersensitivity to any of its ingredients, or to loratadine
- Patients with narrow-angle glaucoma
- Patients with urinary retention
- Patients receiving monoamine oxidase (MAO) inhibitor therapy or within fourteen (14) days of stopping such treatment
- Patients with severe hypertension or severe coronary artery disease
4ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Cardiovascular and Central Nervous System effects
- Increased intraocular pressure
- Urinary retention in patients with prostatic hypertrophy
- Hypersensitivity reactions
- Severe Skin Reactions
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety data described below are from 2 clinical trials with CLARINEX-D 12 HOUR Extended Release Tablets that included 1248 patients with seasonal allergic rhinitis, of which 414 patients received CLARINEX-D 12 HOUR Extended Release Tablets twice daily for up to 2 weeks. The majority of patients were between 18 and <65 years of age with a mean age of 35.8 years and were predominantly women (64%). Patient ethnicity was 82% Caucasian, 9% Black, 6% Hispanic and 3% Asian/other ethnicity. The percentage of subjects receiving CLARINEX-D 12 HOUR Extended Release Tablets and who discontinued from the clinical trials because of an adverse event was 3.6%. Adverse reactions that were reported by ≥2% of subjects receiving CLARINEX-D 12 HOUR Extended Release Tablets are shown in
There were no relevant differences in adverse reactions for subgroups of patients as defined by gender, age, or race.
4.2Post-Marketing Experience
In addition to the adverse reactions reported during clinical trials and listed above, adverse events have been identified during post approval use of CLARINEX-D 12 HOUR Extended Release Tablets. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse events identified from post-marketing surveillance on the use of CLARINEX-D 12 HOUR Extended Release Tablets include:
In addition to these events, the following spontaneous adverse events have been reported during the marketing of desloratadine as a single ingredient product:
Cases of severe skin reactions such as acute generalized exanthematous pustulosis (AGEP) have been reported with pseudoephedrine-containing products.
5DRUG INTERACTIONS
No specific interaction studies have been conducted with CLARINEX-D 12 HOUR Extended Release Tablets.
5.1Monoamine Oxidase Inhibitors
CLARINEX-D 12 HOUR Extended Release Tablets should not be used in patients receiving monoamine oxidase (MAO) inhibitor therapy or within fourteen (14) days of stopping such treatment because the action of pseudoephedrine a component of CLARINEX-D 12 HOUR Extended Release tablets on the vascular system may be potentiated by these agents
5.2Beta-Adrenergic Blocking Agents
The antihypertensive effects of beta-adrenergic blocking agents, methyldopa, and reserpine, may be reduced by sympathomimetics such as pseudoephedrine. Exercise caution when using CLARINEX-D 12 HOUR Extended Release Tablets with these agents.
5.3Digitalis
Increased ectopic pacemaker activity can occur when pseudoephedrine is used concomitantly with digitalis. Exercise caution when using CLARINEX-D 12 HOUR Extended Release Tablets with these agents.
5.4Inhibitors of Cytochrome P450 3A4
In controlled clinical studies co-administration of desloratadine with ketoconazole, erythromycin, or azithromycin resulted in increased plasma concentrations of desloratadine and 3-hydroxydesloratadine but there were no clinically relevant changes in the safety profile of desloratadine
5.5Fluoxetine
In controlled clinical studies co-administration of desloratadine with fluoxetine, a selective serotonin reuptake inhibitor (SSRI), resulted in increased plasma concentrations of desloratadine and 3-hydroxydesloratadine but there were no clinically relevant changes in the safety profile of desloratadine
5.6Cimetidine
In controlled clinical studies co-administration of desloratadine with cimetidine a histamine H
6DRUG ABUSE AND DEPENDENCE
There is no information to indicate that abuse or dependency occurs with CLARINEX or CLARINEX-D 12 HOUR Extended Release Tablets.
7OVERDOSAGE
In the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended. Desloratadine and 3-hydroxydesloratadine are not eliminated by hemodialysis.
7.1Desloratadine
Information regarding acute overdosage with desloratadine is limited to experience from post-marketing adverse event reports and from clinical trials conducted during the development of the CLARINEX product. In the reported cases of overdose, there were no significant adverse events that were attributed to desloratadine. In a dose-ranging trial, at doses of 10 mg and 20 mg/day, somnolence was reported.
In another study, no clinically relevant adverse events were reported in normal male and female volunteers who were given single daily doses of CLARINEX 45 mg for 10 days
7.2Sympathomimetics
In large doses, sympathomimetics such as pseudoephedrine may give rise to giddiness, headache, nausea, vomiting, sweating, thirst, tachycardia, precordial pain, palpitations, difficulty in micturition, muscle weakness and tenseness, anxiety, restlessness, and insomnia. Many patients can present a toxic psychosis with delusions and hallucinations. Some may develop cardiac arrhythmias, circulatory collapse, convulsions, coma, and respiratory failure.
8DESCRIPTION
CLARINEX-D 12 HOUR Extended Release Tablets are oval-shaped blue and white bilayer tablets containing 2.5 mg desloratadine in the blue immediate-release layer and 120 mg of pseudoephedrine sulfate USP in the white extended-release layer which is released slowly, allowing for twice-daily administration.
The inactive ingredients contained in CLARINEX-D 12 HOUR Extended Release Tablets are hypromellose USP, microcrystalline cellulose NF, povidone USP, silicon dioxide NF, magnesium stearate NF, corn starch NF, edetate disodium USP, citric acid anhydrous USP, stearic acid NF, and FD&C Blue No. 2 aluminum lake dye.
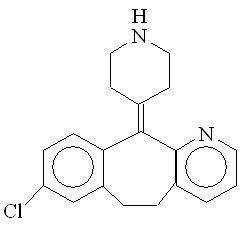
Desloratadine, 1 of the 2 active ingredients of CLARINEX-D 12 HOUR Extended Release Tablets, is a white to off-white powder that is slightly soluble in water, but very soluble in ethanol and propylene glycol. It has an empirical formula: C
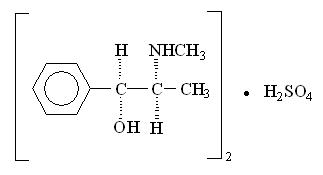
Pseudoephedrine sulfate, the other active ingredient of CLARINEX-D 12 HOUR Extended Release Tablets, is the synthetic salt of one of the naturally occurring dextrorotatory diastereomers of ephedrine and is classified as an indirect sympathomimetic amine. Pseudoephedrine sulfate is a colorless hygroscopic crystal or white, hygroscopic crystalline powder, practically odorless, with a bitter taste. It is very soluble in water, freely soluble in alcohol, and sparingly soluble in ether. The empirical formula for pseudoephedrine sulfate is (C
9HOW SUPPLIED/STORAGE AND HANDLING
CLARINEX-D 12 HOUR Extended Release Tablets are oval-shaped, blue and white bilayer tablets with "D12" embossed in the blue layer, containing 2.5 mg desloratadine in the blue immediate-release layer and 120 mg of pseudoephedrine sulfate USP in the white extended-release layer. CLARINEX-D 12 HOUR Extended Release Tablets are supplied in high-density polyethylene bottles of 100 (NDC 78206-120-01).
10PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
10.1Cardiovascular and Central Nervous System Effects
Patients should be informed that pseudoephedrine, one of the active ingredients in CLARINEX-D 12 HOUR Extended Release Tablets may cause cardiovascular or central nervous system effects such as insomnia, dizziness, tremor, convulsions or arrhythmia.
10.2Dosing
Patients should be advised not to increase the dose or dosing frequency of CLARINEX-D 12 HOUR Extended Release Tablets.
10.3Additional Antihistamines and/or Decongestants
Patients should be advised against the concurrent use of CLARINEX-D 12 HOUR Extended Release Tablets with other antihistamines and/or decongestants.
10.4Monoamine Oxidase (MAO) Inhibitors
Patients should be informed that due to its pseudoephedrine component, they should not use CLARINEX-D 12 HOUR with a monoamine oxidase (MAO) inhibitor or within 14 days of stopping use of an MAO inhibitor.
10.5Coexisting Conditions
Patients with severe hypertension or severe coronary artery disease, narrow-angle glaucoma, or urinary retention should be advised not to use CLARINEX-D 12 HOUR Extended Release Tablets.
10.6Instructions for Use
Patients should be instructed not to break, crush, or chew the tablet; the tablet should be swallowed whole, and can be taken without regard to meals.
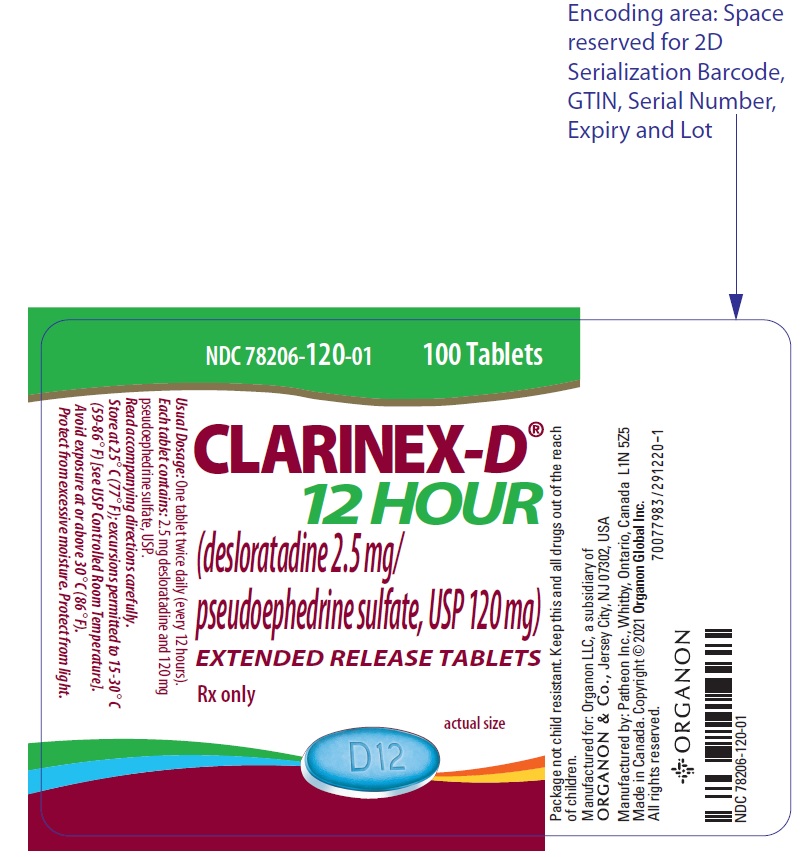
11PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
NDC 78206-120-01
CLARINEX
(desloratadine 2.5 mg/
(desloratadine 2.5 mg/
EXTENDED RELEASE TABLETS
Rx only
actual size